当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Dots as Green Corrosion Inhibitor for Mild Steel in HCl Solution
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202000625 Vandana Saraswat 1 , Mahendra Yadav 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202000625 Vandana Saraswat 1 , Mahendra Yadav 1
Affiliation
|
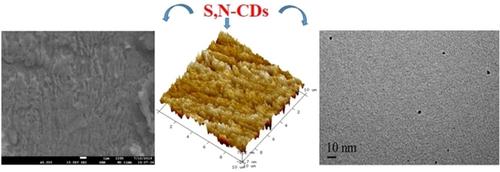
Two carbon dots namely, S, N co‐doped (CD1) and N doped (CD2) were synthesized by solvothermal treatment of pyromelletic acid in presence of thiourea, urea and DETA at 180 °C. The synthesized carbon dots were characterized by Fourier Transform Infrared Spectroscopy (FTIR), Transmission electron microscopy (TEM) and Raman spectroscopy analysis. The dimension of synthesized carbon dots were found in the range of 1.63 nm to 2 nm with significant graphitic carbons. These carbon dots (CD1 and CD2) were used as green corrosion inhibitor to mitigate corrosion of mild steel (MS) in 15 % HCl solution using gravimetric and electrochemical methods. Studied carbon dots, CD1 and CD2 exhibited inhibition efficiency of 96.40 and 90.00 %, respectively, at 100 ppm concentration and 303 K temperature. The observed corrosion inhibition occurs due to adsorption of the carbon dots to the MS surface. Both the carbon dots follow Langmuir adsorption isotherm model and show physisorption on the MS surface. Atomic force microscopy (AFM) and scanning electron microscopy (SEM) analysis was used to study the morphology of the uninhibited and inhibited surface of the sample. The interaction of the carbon dots and composition of adsorbed layer on the MS surface was confirmed using X‐ray photoelectron spectroscopy (XPS). The XPS analysis revealed that heteroatoms present in the structural moiety of the carbon dots efficiently binds on the MS surface.
中文翻译:

碳点作为HCl溶液中低碳钢的绿色缓蚀剂
通过在180°C的硫脲,尿素和DETA存在下对均苯四酸进行溶剂热处理,合成了两个碳点,即S,N共掺杂(CD1)和N掺杂(CD2)。通过傅立叶变换红外光谱(FTIR),透射电子显微镜(TEM)和拉曼光谱分析对合成的碳点进行表征。发现合成碳点的尺寸在1.63 nm至2 nm范围内,且石墨碳含量较高。这些碳点(CD1和CD2)用作绿色腐蚀抑制剂,可通过重量分析法和电化学方法减轻15%HCl溶液中低碳钢(MS)的腐蚀。研究的碳点CD1和CD2在100 ppm浓度和303 K温度下的抑制效率分别为96.40和90.00%。观察到的腐蚀抑制是由于碳点吸附到MS表面而发生的。两个碳点均遵循Langmuir吸附等温线模型,并在MS表面显示出物理吸附。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。
更新日期:2020-07-01
中文翻译:

碳点作为HCl溶液中低碳钢的绿色缓蚀剂
通过在180°C的硫脲,尿素和DETA存在下对均苯四酸进行溶剂热处理,合成了两个碳点,即S,N共掺杂(CD1)和N掺杂(CD2)。通过傅立叶变换红外光谱(FTIR),透射电子显微镜(TEM)和拉曼光谱分析对合成的碳点进行表征。发现合成碳点的尺寸在1.63 nm至2 nm范围内,且石墨碳含量较高。这些碳点(CD1和CD2)用作绿色腐蚀抑制剂,可通过重量分析法和电化学方法减轻15%HCl溶液中低碳钢(MS)的腐蚀。研究的碳点CD1和CD2在100 ppm浓度和303 K温度下的抑制效率分别为96.40和90.00%。观察到的腐蚀抑制是由于碳点吸附到MS表面而发生的。两个碳点均遵循Langmuir吸附等温线模型,并在MS表面显示出物理吸附。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。原子力显微镜(AFM)和扫描电子显微镜(SEM)分析用于研究样品的未抑制和抑制表面的形态。使用X射线光电子能谱(XPS)证实了碳点与MS表面吸附层组成的相互作用。XPS分析表明,碳点结构部分中存在的杂原子有效地结合在MS表面上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号