当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Aldol Reaction of Pyruvate Promoted by Chiral Tertiary Amines
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001450 Monika Pasternak‐Suder 1 , Wojciech Pacułt 1 , Sebastian Baś 1 , Jacek Mlynarski 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001450 Monika Pasternak‐Suder 1 , Wojciech Pacułt 1 , Sebastian Baś 1 , Jacek Mlynarski 2
Affiliation
|
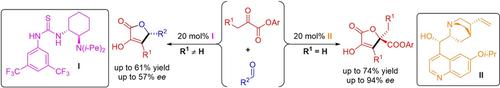
The direct asymmetric aldol reaction of α‐ketoesters catalyzed by chiral tertiary amines is reported. The described methodology is characterized by mild reaction conditions and distinct product selectivity determined by the starting materials. In the developed transformation pyruvates undergo highly selective self‐condensation reaction, whereas cross‐aldol reaction take place predominantly for their carbon chain homologues in presence of aldehyde acceptors. Notably, addition of bulky phenol moiety into pyruvate inhibit the spontaneous lactonization of products and enhance the enantioselectivity.
中文翻译:

手性叔胺促进丙酮酸丙酮酸酯的不对称羟醛反应
报道了手性叔胺催化α-酮酸酯的直接不对称醛醇缩合反应。所描述的方法的特征在于温和的反应条件和由原料确定的独特的产物选择性。在发达的转化中,丙酮酸经历高度选择性的自缩合反应,而交叉醛缩反应主要是由于醛受体存在下的碳链同系物而发生的。值得注意的是,将大量的酚部分添加到丙酮酸中会抑制产物的自发内酯化并增强对映选择性。
更新日期:2020-07-01
中文翻译:

手性叔胺促进丙酮酸丙酮酸酯的不对称羟醛反应
报道了手性叔胺催化α-酮酸酯的直接不对称醛醇缩合反应。所描述的方法的特征在于温和的反应条件和由原料确定的独特的产物选择性。在发达的转化中,丙酮酸经历高度选择性的自缩合反应,而交叉醛缩反应主要是由于醛受体存在下的碳链同系物而发生的。值得注意的是,将大量的酚部分添加到丙酮酸中会抑制产物的自发内酯化并增强对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号