当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Colorimetric Detection of Copper(II) Ions Using Schiff‐Base Derivatives
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001041 Ziya Aydin 1 , Mustafa Keles 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/slct.202001041 Ziya Aydin 1 , Mustafa Keles 2
Affiliation
|
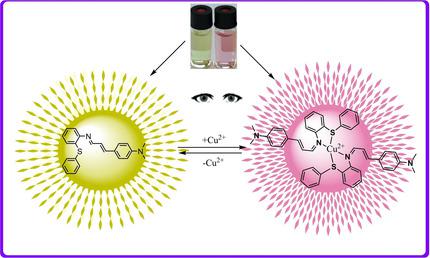
Two new Schiff base derivatives N,N‐dimethyl‐4‐((2‐(phenylthio) phenyl)imino)propenyl)aniline (CSP ) and 2‐(4‐(dimethylamino)phenyl) allylidene) amino)benzenethiol (CSH ) were synthesized. Sensing mechanism of the sensors was investigated using UV‐Vis spectroscopy in the presence of various metal ions. The sensor CSP shows high sensitivity and selectivity to Cu2+ over a wide range of other metal ions in acetonitrile (ACN)/water (H2O) (v/v 2 : 1). The sensor CSP can detect Cu2+ ions by color change from yellow to pink. The complex stoichiometry between the sensor, CSP , and Cu2+ was found to be 2 : 1 and the binding constant was calculated to be 2.40 × 1010 M−2. The absorbance detection limit of CSP for Cu2+ was determined as 2.85 × 10−6 M. The sensor, CSP , was also applied to real samples successfully.
中文翻译:

使用席夫碱衍生物比色检测铜离子
两个新的席夫碱衍生物N,N-二甲基-4-(((2-(苯硫基)苯基)亚氨基)丙烯基)苯胺)苯胺(CSP)和2-(4-(二甲基氨基)苯基)亚芳基)氨基)苯硫醇(CSH)合成的。在各种金属离子存在的情况下,使用紫外可见光谱研究了传感器的传感机理。传感器CSP在乙腈(ACN)/水(H 2 O)(v / v 2:1)中对多种其他金属离子显示出对Cu 2+的高灵敏度和选择性。传感器CSP可以通过从黄色变为粉红色的颜色来检测Cu 2+离子。传感器,CSP和Cu 2+之间的复杂化学计量测得的结合常数为2∶1,结合常数经计算为2.40×10 10 M -2。的吸光度检测限CSP对Cu 2+被确定为2.85×10 -6 M的传感器,CSP,也施加到实际样品成功。
更新日期:2020-07-01
中文翻译:

使用席夫碱衍生物比色检测铜离子
两个新的席夫碱衍生物N,N-二甲基-4-(((2-(苯硫基)苯基)亚氨基)丙烯基)苯胺)苯胺(CSP)和2-(4-(二甲基氨基)苯基)亚芳基)氨基)苯硫醇(CSH)合成的。在各种金属离子存在的情况下,使用紫外可见光谱研究了传感器的传感机理。传感器CSP在乙腈(ACN)/水(H 2 O)(v / v 2:1)中对多种其他金属离子显示出对Cu 2+的高灵敏度和选择性。传感器CSP可以通过从黄色变为粉红色的颜色来检测Cu 2+离子。传感器,CSP和Cu 2+之间的复杂化学计量测得的结合常数为2∶1,结合常数经计算为2.40×10 10 M -2。的吸光度检测限CSP对Cu 2+被确定为2.85×10 -6 M的传感器,CSP,也施加到实际样品成功。











































 京公网安备 11010802027423号
京公网安备 11010802027423号