当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-Catalyzed Rearrangements of 3-Aryloxirane-2-Carboxamides: Novel DFT Mechanistic Insights.
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-07-01 , DOI: 10.1002/open.202000110 Zheng-Wang Qu 1 , Hui Zhu 1 , Sergey A Katsyuba 2 , Vera L Mamedova 2 , Vakhid A Mamedov 2 , Stefan Grimme 1
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-07-01 , DOI: 10.1002/open.202000110 Zheng-Wang Qu 1 , Hui Zhu 1 , Sergey A Katsyuba 2 , Vera L Mamedova 2 , Vakhid A Mamedov 2 , Stefan Grimme 1
Affiliation
|
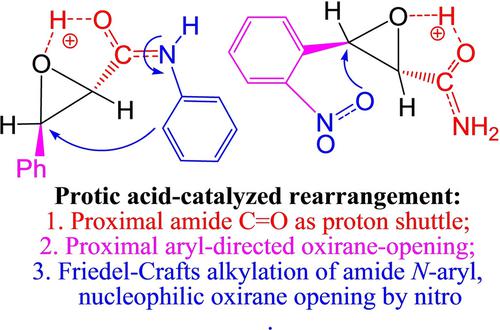
Efficient synthesis of 3‐arylquinolin‐2(1H)‐ones and N‐(2‐carboxyaryl)‐oxalamides from protic acid‐catalyzed rearrangements of 3‐aryloxirane‐2‐carboxamides was achieved recently but not well understood. In contrast to the classical Meinwald rearrangement, extensive DFT calculations reveal that the proximal aryl and amide groups have strong synergetic effects to control the amide‐aided and aryl‐directed oxirane‐opening and further rearrangement sequences. The ortho‐nitro substituent of the proximal aryl is directly involved in a nucleophilic oxirane ring‐opening, the amide C=O is an important proton shuttle for facile H‐shifts, while the N‐aryl may act as a potential ring‐closing site via Friedel‐Crafts alkylation. The mechanistic insights are useful for rational design of novel synthesis by changing the aryl and amide functional groups proximal to the oxirane ring.
中文翻译:

3-芳基环氧乙烷-2-甲酰胺的酸催化重排:新颖的 DFT 机理见解。
最近通过质子酸催化的 3-芳基环氧乙烷-2-甲酰胺重排实现了 3-芳基喹啉-2(1 H )-酮和N- (2-羧芳基)-草酰胺的高效合成,但尚未得到充分理解。与经典的Meinwald重排相反,广泛的DFT计算表明,近端芳基和酰胺基团具有很强的协同效应,可以控制酰胺辅助和芳基定向的环氧乙烷开环和进一步重排序列。近端芳基的邻硝基取代基直接参与亲核环氧乙烷开环,酰胺C=O是重要的质子穿梭,可实现轻松的H-位移,而N-芳基可以作为潜在的闭环位点通过弗里德尔-克来福特烷基化。通过改变环氧乙烷环附近的芳基和酰胺官能团,该机制的见解对于合理设计新型合成非常有用。
更新日期:2020-07-01
中文翻译:

3-芳基环氧乙烷-2-甲酰胺的酸催化重排:新颖的 DFT 机理见解。
最近通过质子酸催化的 3-芳基环氧乙烷-2-甲酰胺重排实现了 3-芳基喹啉-2(1 H )-酮和N- (2-羧芳基)-草酰胺的高效合成,但尚未得到充分理解。与经典的Meinwald重排相反,广泛的DFT计算表明,近端芳基和酰胺基团具有很强的协同效应,可以控制酰胺辅助和芳基定向的环氧乙烷开环和进一步重排序列。近端芳基的邻硝基取代基直接参与亲核环氧乙烷开环,酰胺C=O是重要的质子穿梭,可实现轻松的H-位移,而N-芳基可以作为潜在的闭环位点通过弗里德尔-克来福特烷基化。通过改变环氧乙烷环附近的芳基和酰胺官能团,该机制的见解对于合理设计新型合成非常有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号