当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Iodine(III)-Promoted Oxidative Dearomatizing Hydroxylation of Phenols: Evidence for a Radical-Chain Pathway.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/chem.202002026 Karol Kraszewski 1, 2 , Ireneusz Tomczyk 1, 2 , Aneta Drabinska 3 , Krzysztof Bienkowski 1 , Renata Solarska 1 , Marcin Kalek 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-07-01 , DOI: 10.1002/chem.202002026 Karol Kraszewski 1, 2 , Ireneusz Tomczyk 1, 2 , Aneta Drabinska 3 , Krzysztof Bienkowski 1 , Renata Solarska 1 , Marcin Kalek 1
Affiliation
|
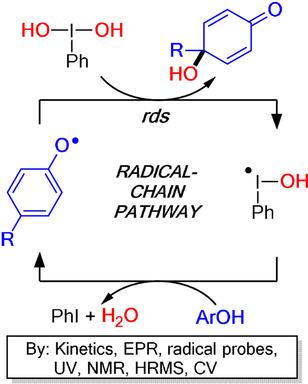
The oxidative dearomatization of phenols with the addition of nucleophiles to the aromatic ring induced by hypervalent iodine(III) reagents and catalysts has emerged as a highly useful synthetic approach. However, experimental mechanistic studies of this important process have been extremely scarce. In this report, we describe systematic investigations of the dearomatizing hydroxylation of phenols using an array of experimental techniques. Kinetics, EPR spectroscopy, and reactions with radical probes demonstrate that the transformation proceeds by a radical‐chain mechanism, with a phenoxyl radical being the key chain‐carrying intermediate. Moreover, UV and NMR spectroscopy, high‐resolution mass spectrometry, and cyclic voltammetry show that before reacting with the phenoxyl radical, the water molecule becomes activated by the interaction with the iodine(III) center, causing the Umpolung of this formally nucleophilic substrate. The radical‐chain mechanism allows the rationalization of all existing observations regarding the iodine(III)‐promoted oxidative dearomatization of phenols.
中文翻译:

碘(III)促进苯酚的氧化脱芳香化羟基化的机理:自由基链途径的证据。
作为一种非常有用的合成方法,已经出现了在高价碘(III)试剂和催化剂诱导的芳香环中添加亲核试剂的酚类氧化脱芳香化反应。但是,对这一重要过程的实验机制研究极为匮乏。在这份报告中,我们描述了使用一系列实验技术对苯酚进行脱芳香化羟基化的系统研究。动力学,EPR光谱学以及与自由基探针的反应表明,这种转变是通过自由基链机制进行的,苯氧基自由基是携带关键链的中间体。此外,UV和NMR光谱,高分辨率质谱和循环伏安法表明,在与苯氧基自由基反应之前,水分子通过与碘(III)中心的相互作用而被激活,从而导致这种形式的亲核底物形成Umpolung。自由基链机制可合理化所有有关碘(III)促进酚氧化脱芳香化的现有观察结果。
更新日期:2020-09-05
中文翻译:

碘(III)促进苯酚的氧化脱芳香化羟基化的机理:自由基链途径的证据。
作为一种非常有用的合成方法,已经出现了在高价碘(III)试剂和催化剂诱导的芳香环中添加亲核试剂的酚类氧化脱芳香化反应。但是,对这一重要过程的实验机制研究极为匮乏。在这份报告中,我们描述了使用一系列实验技术对苯酚进行脱芳香化羟基化的系统研究。动力学,EPR光谱学以及与自由基探针的反应表明,这种转变是通过自由基链机制进行的,苯氧基自由基是携带关键链的中间体。此外,UV和NMR光谱,高分辨率质谱和循环伏安法表明,在与苯氧基自由基反应之前,水分子通过与碘(III)中心的相互作用而被激活,从而导致这种形式的亲核底物形成Umpolung。自由基链机制可合理化所有有关碘(III)促进酚氧化脱芳香化的现有观察结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号