当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human Cellular Retinol Binding Protein II Forms a Domain-Swapped Trimer Representing a Novel Fold and a New Template for Protein Engineering.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-06-30 , DOI: 10.1002/cbic.202000405 Alireza Ghanbarpour 1, 2 , Elizabeth M Santos 1, 3 , Cody Pinger 4 , Zahra Assar 5 , Seyedmehdi Hossaini Nasr 1 , Chrysoula Vasileiou 1 , Dana Spence 4 , Babak Borhan 1 , James H Geiger 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-06-30 , DOI: 10.1002/cbic.202000405 Alireza Ghanbarpour 1, 2 , Elizabeth M Santos 1, 3 , Cody Pinger 4 , Zahra Assar 5 , Seyedmehdi Hossaini Nasr 1 , Chrysoula Vasileiou 1 , Dana Spence 4 , Babak Borhan 1 , James H Geiger 1
Affiliation
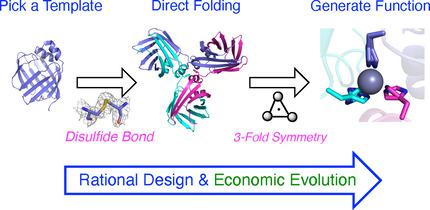
|
Protein fold control: The domain‐swapping folding pathway of human cellular retinol binding protein II has been controlled, and the resulting domain‐swapped trimer made into a metal binding protein, with a minimum of amino acid changes, by using structure‐guided protein design. This illustrates maximally economic evolution of both structure and function.

中文翻译:

人细胞视黄醇结合蛋白 II 形成结构域交换的三聚体,代表一种新的折叠和蛋白质工程的新模板。
蛋白质折叠控制:通过使用结构引导的蛋白质设计,控制了人细胞视黄醇结合蛋白II的结构域交换折叠途径,并将所得的结构域交换三聚体制成金属结合蛋白,氨基酸变化最少。这说明了结构和功能的最大经济演变。

更新日期:2020-06-30

中文翻译:

人细胞视黄醇结合蛋白 II 形成结构域交换的三聚体,代表一种新的折叠和蛋白质工程的新模板。
蛋白质折叠控制:通过使用结构引导的蛋白质设计,控制了人细胞视黄醇结合蛋白II的结构域交换折叠途径,并将所得的结构域交换三聚体制成金属结合蛋白,氨基酸变化最少。这说明了结构和功能的最大经济演变。












































 京公网安备 11010802027423号
京公网安备 11010802027423号