当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fig. TOC, methylammonium dicyanamide: Synthesis, characterisation, and screening its potential as a precursor for C3N4 and as an ionic liquid
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.solidstatesciences.2020.106347 Carsten L. Schmidt , Jürgen Nuss , Martin Jansen
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.solidstatesciences.2020.106347 Carsten L. Schmidt , Jürgen Nuss , Martin Jansen
|
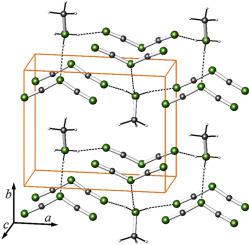
Abstract Methylammonium dicyanamide, (CH3NH3)[N(CN)2], was synthesized by metathesis from methylammonium chloride and silver dicyanamide, Ag [N(CN)2], in dry diethyl ether. The crystal structure was solved from single-crystal X-ray data at T = −173 °C (Pma2, a = 8.9541 (11) A, b = 7.5136 (9) A, c = 3.9631 (5) A; V = 266.63 (6) A3; Z = 2). The title compound was further analysed by means of NMR and Raman spectroscopies as well as DSC and DTA. Methylammonium dicyanamide, a waxy solid at RT, may be classified as an ionic liquid at T > 63 °C. At lower temperatures, methylammonium dicyanamide exhibits a robust network of hydrogen bonds, stabilizing and fixing the molecular cations as well as anions in the crystal lattice. Above T ~43 °C, indications for an onset of dynamic disorder are discernible. Finally, beyond T = 170 °C, the initially salt-like compound decomposes by releasing stable and volatile molecules (e.g. H2NCN, HCN, NH3). The title compound, C3N4H6, displaying a C/N ratio of ¾, is a potential single source precursor for the synthesis of C3N4, and fulfils the commonly accepted criteria to be addressed as an “ionic liquid”.
中文翻译:

图 TOC,二氰胺甲基铵:合成、表征和筛选其作为 C3N4 前体和离子液体的潜力
摘要 甲基二氰胺(CH3NH3)[N(CN)2]是由甲基氯化铵和二氰胺银Ag[N(CN)2]在无水乙醚中复分解合成的。晶体结构由 T = -173 °C 时的单晶 X 射线数据求解(Pma2, a = 8.9541 (11) A, b = 7.5136 (9) A, c = 3.9631 (5) A; V = 266.63 (6) A3;Z = 2)。通过NMR和拉曼光谱以及DSC和DTA进一步分析标题化合物。二氰胺甲基铵在室温下为蜡状固体,在 T > 63 °C 时可归类为离子液体。在较低温度下,二氰胺甲基铵表现出强大的氢键网络,稳定和固定分子阳离子以及晶格中的阴离子。高于 T ~43 °C,动态障碍发作的迹象是可辨别的。最后,超过 T = 170 °C,最初的盐状化合物通过释放稳定且易挥发的分子(例如 H2NCN、HCN、NH3)而分解。标题化合物 C3N4H6 的 C/N 比为 3/4,是合成 C3N4 的潜在单一来源前体,符合公认的“离子液体”标准。
更新日期:2020-09-01
中文翻译:

图 TOC,二氰胺甲基铵:合成、表征和筛选其作为 C3N4 前体和离子液体的潜力
摘要 甲基二氰胺(CH3NH3)[N(CN)2]是由甲基氯化铵和二氰胺银Ag[N(CN)2]在无水乙醚中复分解合成的。晶体结构由 T = -173 °C 时的单晶 X 射线数据求解(Pma2, a = 8.9541 (11) A, b = 7.5136 (9) A, c = 3.9631 (5) A; V = 266.63 (6) A3;Z = 2)。通过NMR和拉曼光谱以及DSC和DTA进一步分析标题化合物。二氰胺甲基铵在室温下为蜡状固体,在 T > 63 °C 时可归类为离子液体。在较低温度下,二氰胺甲基铵表现出强大的氢键网络,稳定和固定分子阳离子以及晶格中的阴离子。高于 T ~43 °C,动态障碍发作的迹象是可辨别的。最后,超过 T = 170 °C,最初的盐状化合物通过释放稳定且易挥发的分子(例如 H2NCN、HCN、NH3)而分解。标题化合物 C3N4H6 的 C/N 比为 3/4,是合成 C3N4 的潜在单一来源前体,符合公认的“离子液体”标准。











































 京公网安备 11010802027423号
京公网安备 11010802027423号