Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.omtm.2020.06.024 Janice M Riberdy 1 , Sheng Zhou 2 , Fei Zheng 2 , Young-In Kim 2 , Jennifer Moore 1 , Abishek Vaidya 1 , Robert E Throm 3 , April Sykes 4 , Natasha Sahr 4 , Challice L Bonifant 5 , Byoung Ryu 3 , Stephen Gottschalk 1 , Mireya Paulina Velasquez 1
|
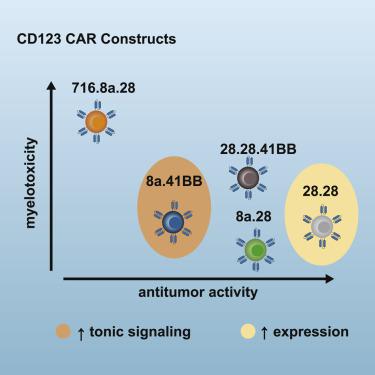
Chimeric antigen receptor (CAR) T cells targeting CD123, an acute myeloid leukemia (AML) antigen, hold the promise of improving outcomes for patients with refractory/recurrent disease. We generated five lentiviral vectors encoding CD20, which may serve as a target for CAR T cell depletion, and 2nd or 3rd generation CD123-CARs since the benefit of two costimulatory domains is model dependent. Four CARs were based on the CD123-specific single-chain variable fragment (scFv) 26292 (292) and one CAR on the CD123-specific scFv 26716 (716), respectively. We designed CARs with different hinge/transmembrane (H/TM) domains and costimulatory domains, in combination with the zeta (z) signaling domain: 292.CD8aH/TM.41BBz (8.41BBz), 292.CD8aH/TM.CD28z (8.28z), 716.CD8aH/TM.CD28z (716.8.28z), 292.CD28H/TM. CD28z (28.28z), and 292.CD28H/TM.CD28.41BBz (28.28.41BBz). Transduction efficiency, expansion, phenotype, and target cell recognition of the generated CD123-CAR T cells did not significantly differ. CAR constructs were eliminated for the following reasons: (1) 8.41BBz CARs induced significant baseline signaling, (2) 716.8.28z CAR T cells had decreased anti-AML activity, and (3) CD28.41BBz CAR T cells had no improved effector function in comparison to CD28z CAR T cells. We selected the 28.28z CAR since CAR expression on the cell surface of transduced T cells was higher in comparison to 8.28z CARs. The clinical study (NCT04318678) evaluating 28.28z CAR T cells is now open for patient accrual.
中文翻译:

选择 CD123 特异性嵌合抗原受体进行临床测试的艺术和科学。
靶向 CD123(一种急性髓系白血病 (AML) 抗原)的嵌合抗原受体 (CAR) T 细胞有望改善难治性/复发性疾病患者的治疗结果。我们生成了五个编码 CD20 的慢病毒载体(可作为 CAR T 细胞耗竭的靶标)和第二代或第三代 CD123-CAR,因为两个共刺激域的益处取决于模型。四种 CAR 分别基于 CD123 特异性单链可变片段 (scFv) 26292 (292),一种 CAR 基于 CD123 特异性 scFv 26716 (716)。我们设计了具有不同铰链/跨膜 (H/TM) 结构域和共刺激结构域的 CAR,并结合 zeta (z) 信号结构域:292.CD8aH/TM.41BBz (8.41BBz)、292.CD8aH/TM.CD28z (8.28) z)、716.CD8aH/TM.CD28z(716.8.28z)、292.CD28H/TM。 CD28z (28.28z) 和 292.CD28H/TM.CD28.41BBz (28.28.41BBz)。生成的 CD123-CAR T 细胞的转导效率、扩增、表型和靶细胞识别没有显着差异。 CAR 构建体被淘汰的原因如下:(1) 8.41BBz CAR 诱导显着的基线信号传导,(2) 716.8.28z CAR T 细胞的抗 AML 活性降低,(3) CD28.41BBz CAR T 细胞没有改善的效应子与 CD28z CAR T 细胞相比,其功能更强。我们选择 28.28z CAR,因为转导 T 细胞的细胞表面上的 CAR 表达高于 8.28z CAR。评估 28.28z CAR T 细胞的临床研究 (NCT04318678) 现已开放供患者招募。











































 京公网安备 11010802027423号
京公网安备 11010802027423号