Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.omtm.2020.06.025 Qingnan Wang 1 , Xiaomei Zhong 1 , Qian Li 1 , Jing Su 1 , Yi Liu 1 , Li Mo 1 , Hongxin Deng 1 , Yang Yang 1
|
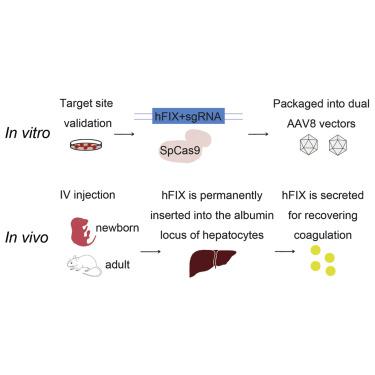
Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 loaded by vectors could induce high rates of specific site genome editing and correct disease-causing mutations. However, most monogenic genetic diseases such as hemophilia are caused by different mutations dispersed in one gene, instead of an accordant mutation. Vectors developed for correcting specific mutations may not be suited to different mutations at other positions. Site-specific gene addition provides an ideal solution for long-term, stable gene therapy. We have demonstrated SaCas9-mediated homology-directed factor IX (FIX) in situ targeting for sustained treatment of hemophilia B. In this study, we tested a more efficient dual adeno-associated virus (AAV) strategy with lower vector dose for liver-directed genome editing that enables CRISPR-Cas9-mediated site-specific integration of therapeutic transgene within the albumin gene, and we aimed to develop a more universal gene-targeting approach. We successfully achieved coagulation function in newborn and adult hemophilia B mice by a single injection of dual AAV vectors. FIX levels in treated mice persisted even after a two-thirds partial hepatectomy, indicating stable gene integration. Our results suggest that this CRISPR-Cas9-mediated site-specific gene integration in hepatocytes could transform into a common clinical therapeutic method for hemophilia B and other genetic diseases.
中文翻译:

白蛋白基因座处的CRISPR-Cas9介导的体内基因整合可恢复新生儿和成年B型血友病小鼠的止血效果。
载体加载的成簇的规则间隔的短回文重复序列(CRISPR)-Cas9可以诱导高特异性位点基因组编辑率,并纠正引起疾病的突变。但是,大多数单基因遗传病(如血友病)是由分散在一个基因中的不同突变而不是一致的突变引起的。为纠正特定突变而开发的载体可能不适合其他位置的不同突变。位点特异性基因添加为长期,稳定的基因治疗提供了理想的解决方案。我们已经证明SaCas9介导的同源定向因子IX(FIX)原位靶向血友病B的持续治疗。在这项研究中,我们测试了更有效的双腺伴随病毒(AAV)策略,具有较低的载体剂量,可用于肝脏定向基因组编辑,从而实现CRISPR-Cas9介导的治疗性转基因位点特异性整合在白蛋白基因中,我们旨在开发一种更通用的基因靶向方法。通过单次注射双重AAV载体,我们成功地在新生和成年B型血友病小鼠中实现了凝血功能。即使在三分之二的部分肝切除术后,治疗小鼠的FIX水平仍持续存在,表明基因整合稳定。我们的结果表明,这种CRISPR-Cas9介导的肝细胞位点特异性基因整合可以转化为B型血友病和其他遗传疾病的常见临床治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号