Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.jhazmat.2020.123356 Kejia Zhang 1 , Yulong San 1 , Cong Cao 2 , Tuqiao Zhang 1 , Cheng Cen 1 , Zhang Li 3 , Jie Fu 3
|
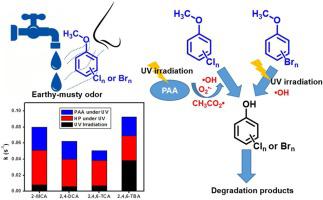
This study reported the kinetics and mechanism of degradation of odorant haloanisoles by peracetic acid combined with UV irradiation (PAA/UV). The removal efficiency of haloanisoles by PAA/UV was more than 92 % after 1 h reaction at pH 5, 25 °C, [HAs] =50 μg/L and [PAA] = 10 mg/L. The degradation of haloanisoles was fitted by the first-order kinetic model, and the rate constants of various haloanisoles followed the order: 2,4,6-tribromoanisole (2,4,6-TBA, (9.25 ± 0.71)×10−2 s-1) > 2-monochloroanisole (2-MCA, (8.00 ± 0.34)×10−2 s-1) > 2,4-dichloroanisole (2,4-DCA, (6.24 ± 0.55)×10−2 s-1) > 2,4,6-trichloroanisole (2,4,6-TCA, (5.05 ± 0.04)×10−2 s-1). The contribution of PAA (mainly composed of free radicals produced from PAA activation by UV) to the degradation rate of chloroanisoles in PAA/UV process ranged from 24 % to 36 %, while 25 % to the degradation rate of bromoanisole. Direct photolysis contributed much more to the removal of bromoanisole (42 %) than chloroanisoles (9–14 %). The inhibition of tert-butanol on degradation demonstrated the existence of ·OH, and superoxide radical and carbon-centered radicals were also probably existed in PAA/UV process. Combining density functional theory (DFT) calculation and products analysis, the degradation pathway of haloanisoles in PAA/UV process were determined. The odor and toxicity evaluation indicated PAA/UV process could reduce olfactory discomfort and health risk of haloanisoles.
中文翻译:

动力学和机理研究过乙酸结合紫外线辐射降解臭味卤苯甲醚的过程。
这项研究报告了过乙酸与紫外线辐射(PAA / UV)结合降解动力学卤代苯甲醚的动力学和机理。在pH 5、25°C,[HAs] = 50μg/ L和[PAA] = 10 mg / L的条件下反应1 h后,PAA / UV去除卤苯甲醚的效率超过92%。用一级动力学模型拟合卤代苯甲醚的降解,各种卤代苯甲醚的速率常数遵循以下顺序:2,4,6-三溴苯甲醚(2,4,6-TBA,(9.25±0.71)×10 -2小号-1)> 2-monochloroanisole(2-MCA,(8.00±0.34)×10 -2小号-1)> 2,4- dichloroanisole(2,4-DCA,(6.24±0.55)×10个-2小号- 1)> 2,4,6-三氯茴香醚(2,4,6-TCA,(5.05±0.04)×10 -2 s-1)。PAA(主要由紫外线激活PAA产生的自由基组成)对PAA / UV过程中氯茴香醚降解率的贡献范围为24%至36%,而对溴苯甲醚降解率的贡献率为25%。直接光解对溴代茴香醚(42%)的去除比氯代茴香醚(9–14%)的去除贡献更大。叔丁醇对降解的抑制作用表明存在·OH,在PAA / UV过程中也可能存在超氧自由基和以碳为中心的自由基。结合密度泛函理论(DFT)计算和产物分析,确定了卤苯甲醚在PAA / UV过程中的降解途径。气味和毒性评估表明,PAA / UV工艺可以减少卤代苯甲醚的嗅觉不适和健康风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号