European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.ejmech.2020.112475 Sai-Sai Xie 1 , Jing Liu 2 , Chunli Tang 3 , Chengyun Pang 3 , Qing Li 3 , Yuelian Qin 3 , Xiaojie Nong 3 , Zhipeng Zhang 4 , Jie Guo 4 , Maojun Cheng 4 , Weizhong Tang 5 , Ningsheng Liang 3 , Neng Jiang 3
|
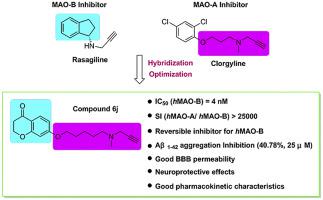
A series of rasagiline-clorgyline hybrids was designed, synthesized and investigated in vitro for their inhibition of monoamine oxidase and amyloid-β aggregation. Most of compounds were found to be selective and highly potent hMAO-B inhibitors showing IC50 values in the nanomolar, and exhibited a moderate inhibition of amyloid-β aggregation. 7-((5-(methyl(prop-2-yn-1-yl)amino) pentyl)oxy)chroman-4-one (6j) was the most interesting compound identified in this research, endowed with higher hMAO-B potency (IC50 = 4 nM) and selectivity (SI > 25000) compared to the reference selective inhibitor rasagiline (IC50 = 141 nM, SI > 355), and exhibited good inhibitory activity against Aβ1-42 aggregation (40.78%, 25 μM). Kinetic and molecular modeling studies revealed that 6j was a competitive reversible inhibitor for hMAO-B. Moreover, compound 6j displayed low toxicity and good neuroprotective effects in SH-SY5Y cell assay, and could penetrate the blood-brain barrier according to the parallel artificial membrane permeability assay. Pharmacokinetics assay revealed that compound 6j possessed good pharmacokinetic profiles after intravenous and oral administrations. Overall, these results highlighted that compound 6j was an effective and promising multitarget agent against Alzheimer’s disease.
中文翻译:

雷沙吉兰-高铁碱杂种作为单胺氧化酶-B和淀粉样β-聚集体对阿尔茨海默氏病的新型双重抑制剂的设计,合成和生物学评估。
设计,合成和合成了一系列雷沙吉兰-氯氰菊酯杂合体,以抑制单胺氧化酶和β-淀粉样蛋白的聚集。发现大多数化合物是选择性的和高效的hMAO-B抑制剂,在纳摩尔中显示IC 50值,并显示出对淀粉样β聚集的中等抑制作用。7-((5-(甲基(prop-2-yn-1-基)氨基)戊基]氧基)苯并二氢吡喃-4-酮(6j)是这项研究中鉴定出的最有趣的化合物,具有较高的hMAO-B效能(IC 50 = 4 nM)和选择性(SI> 25000)与参考选择性抑制剂雷沙吉兰(IC 50 = 141 nM,SI> 355)相比,对Aβ1-42具有良好的抑制活性聚集(40.78%,25μM)。动力学和分子建模研究表明6j是hMAO-B的竞争性可逆抑制剂。此外,化合物6j在SH-SY5Y细胞试验中显示出低毒性和良好的神经保护作用,并且根据平行人工膜通透性试验可以穿透血脑屏障。药代动力学分析表明,化合物6j在静脉和口服给药后均具有良好的药代动力学特征。总体而言,这些结果强调了化合物6j是对抗阿尔茨海默氏病的有效且有前途的多靶点药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号