Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.bioorg.2020.104057 Asad Hamad 1 , Mohsin Abbas Khan 1 , Khondaker Miraz Rahman 2 , Irshad Ahmad 1 , Zaheer Ul-Haq 3 , Samra Khan 4 , Zahid Shafiq 5
|
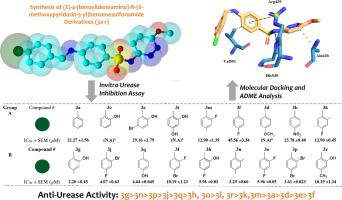
A series of Sulfonamide-based Schiff bases (E)-4-(benzylideneamino)-N-(6-methoxypyridazin-3-yl) benzenesulfonamide (3a-r) targeting Urease Inhibition was synthesized from sulphamethoxy pyridazine and substituted aldehydes. The prepared compounds were characterized by various spectroscopic techniques including FTIR, 1HNMR, 13CNMR, and spectrometric HRMS analysis. The most active agent (3g) bearing halogens and OH groups gave IC50 value of 2.20 µM for urease inhibition against the standard Thiourea (IC50 = 20.03 ± 2.06) and the overall trend within the series was 3g > 3n > 3p > 3j > 3q > 3h, 3o > 3l, 3r > 3k, 3m > 3a > 3d > 3e > 3f. Structure-activity relationship study established that the nature as well as the position of varying groups attached to aryl group had crucial roles in defining the urease inhibition activity. Additionally, in silico investigation was carried out which demonstrated that the compounds exhibit polar and apolar contacts with the crucial residues in the binding site of urease. The ADME analysis suggested all the synthesized compounds to be non-toxic, and likely to undergo passive gastrointestinal absorption. Taken together, the study suggests that the synthesized Sulfonamide-based Schiff bases derivatives may serve as potential hits as urease inhibitors.
中文翻译:

针对脲酶抑制作用的基于磺酰胺的席夫碱的开发:合成,表征,抑制活性评估,分子对接和ADME研究。
从磺胺甲氧基哒嗪和取代的醛类合成了一系列针对脲酶抑制的基于磺酰胺的席夫碱(E)-4-(苄叉氨基)-N-(6-甲氧基吡啶并-3-基)苯磺酰胺(3a-r)。通过各种光谱技术,包括FTIR,1 HNMR,13 CNMR和光谱HRMS分析,对制备的化合物进行表征。带有卤素和OH基团的活性最高的试剂(3g)对标准硫脲的脲酶抑制作用的IC 50值为2.20 µM(IC 50 = 20.03±2.06),并且该系列的总体趋势为3g> 3n> 3p> 3j> 3q> 3h,3o> 3l,3r> 3k,3m> 3a> 3d> 3e> 3f。结构-活性关系研究表明,连接芳基的不同基团的性质和位置在确定脲酶抑制活性方面起着至关重要的作用。另外,进行了计算机分析,证明该化合物与脲酶结合位点中的关键残基表现出极性和非极性接触。ADME分析表明,所有合成的化合物都是无毒的,并且可能会经历被动胃肠吸收。两者合计,研究表明合成的基于磺酰胺的席夫碱衍生物可能作为脲酶抑制剂的潜在打击。











































 京公网安备 11010802027423号
京公网安备 11010802027423号