Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.bioorg.2020.104064 Hongyu Hu 1 , Jun Wu 2 , Mingtao Ao 2 , Xiaoping Zhou 2 , Boqun Li 2 , Zhenzhen Cui 2 , Tong Wu 2 , Lijuan Wang 2 , Yuhua Xue 2 , Zhen Wu 2 , Meijuan Fang 2
|
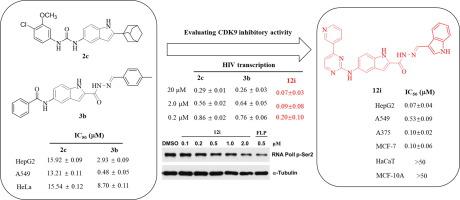
In continuation of our previous work on the investigation of CDK9 inhibitors bearing indole moiety for the discovery of novel anticancer agents, novel methylenehydrazine-1-carboxamide derivatives with (5-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)-1H-indole scaffold were designed, synthesized, and evaluated for the CDK9 inhibitory activity and anticancer activity. Biological activity results demonstrated that most of these derivatives possessed good inhibitory on the kinase activity of CDK9 such as blocking its phosphorylation function and inhibiting HIV-1 transcription. Compound 12i was found to be the most potent CDK9 inhibitor and exhibited excellent anticancer activity against HepG2, A375, MCF-7, and A549, but low toxic on normal cells including HaCaT and MCF-10A. Further studies revealed that as a result of CDK9 inhibition and subsequent inhibition of phosphorylation at Serine 2 of the RNAPII CTD, the representative compound 12i dose-dependently increased cleaved PARP level, exerting its antiproliferative effect through induction of apoptosis in cancer cells. Finally, the molecular docking analysis implied that 12i had a good binding affinity with CDK9. In summary, 12i is a potent CDK9 inhibitor and can be considered as a good lead-candidate for developing potential anticancer drugs.
中文翻译:

具有(5-((4-(吡啶-3-基)嘧啶-2-基)氨基)-1H-吲哚支架)的亚甲基肼-1-甲酰胺衍生物的设计,合成和生物学评估:新型潜在的CDK9抑制剂。
在我们以前的研究中,为了发现新型抗癌药,研究了带有吲哚部分的CDK9抑制剂,即具有(5-((4-(吡啶-3-基)嘧啶-2-基)氨基)-1 H-吲哚支架的设计,合成及对CDK9抑制活性和抗癌活性的评估,生物学活性结果表明,这些衍生物大多数对CDK9的激酶活性具有良好的抑制作用,例如阻断其磷酸化功能和抑制其活性。抑制HIV-1转录化合物12i被发现是最有效的CDK9抑制剂,对HepG2,A375,MCF-7和A549表现出优异的抗癌活性,但对包括HaCaT和MCF-10A在内的正常细胞毒性较低。进一步的研究表明,由于CDK9抑制和随后抑制RNAPII CTD的丝氨酸2磷酸化的结果,代表性化合物12i剂量依赖性地增加了裂解的PARP水平,通过诱导癌细胞凋亡来发挥其抗增殖作用。最后,分子对接分析表明12i与CDK9具有良好的结合亲和力。总之,12i是有效的CDK9抑制剂,可以被认为是开发潜在抗癌药物的良好候选铅。









































 京公网安备 11010802027423号
京公网安备 11010802027423号