Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.bioorg.2020.104053 Ebtehal M Husseiny 1
|
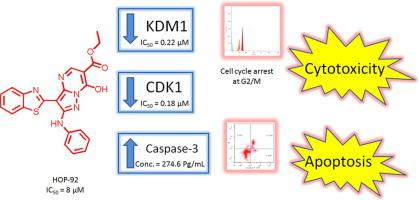
A novel series of pyrazoles and pyrazolo[1,5-a]pyrimidines bearing benzothiazole moiety were designed and synthesized. Chemical structures were confirmed by spectral data and elemental analyses. Nine compounds were selected and screened for their cytotoxic activity at the National Cancer Institute (NCI), USA against 60 cancer cell lines in a single dose assay. Compounds 4 and 5 exerted the most potent growth inhibitory activity against most cancer cell lines with growth inhibition (GI%) ranges from 44.86% to 84.59% and 31.20% to 52.36%, respectively. Consequently, they were further investigated through IC50 determination using five dose MTT colorimetric assay against three sensitive cell lines, leukemia CCRF-CEM, non-small cell lung cancer HOP-92 and liver cancer Hep-G2. Compound 4 exhibited potent cytotoxic activity against the three tested cell lines with IC50 16.34, 3.45 and 7.79 μM, respectively representing half potency, 3.5 folds potency and nearly equipotent to roscovitine. To investigate its mechanism of action, cell cycle analysis of compound 4 was conducted and showed that it induced cell cycle arrest at G2/M phase and apoptosis in HOP-92 cells. In correlation with the previous results, caspase-3 activation was tested and illustrated elevation in its concentration by nearly 14 folds than control. Besides, enzyme inhibition assay of compound 4 was evaluated towards two common antitumor targets namely KDM1 and CDK1 showing significant inhibitory activity with IC50 0.096 and 0.078 μM, respectively.
中文翻译:

某些带有苯并噻唑部分的吡唑和吡唑并[1,5-a]嘧啶的合成,细胞毒性及其作用机理的研究。
设计并合成了一系列新颖的吡唑和带有苯并噻唑部分的吡唑并[1,5- a ]嘧啶。化学结构通过光谱数据和元素分析得到证实。选择了9种化合物,并在美国国家癌症研究所(NCI)进行了单剂量试验,针对60种癌细胞系筛选了它们的细胞毒活性。化合物4和5对大多数癌细胞具有最强的生长抑制活性,生长抑制(GI%)分别为44.86%至84.59%和31.20%至52.36%。因此,他们通过IC 50进行了进一步调查使用五剂量MTT比色测定法对三种敏感细胞系(白血病CCRF-CEM,非小细胞肺癌HOP-92和肝癌Hep-G2)进行测定。化合物4对三种测试的细胞系表现出有效的细胞毒性活性,IC 50为16.34、3.45和7.79μM,分别代表半价,3.5倍效价和对roscovitine几乎等价。为了研究其作用机理,对化合物4进行了细胞周期分析,结果表明它诱导了细胞周期停滞在G2 / M期并诱导了HOP-92细胞凋亡。与以前的结果相关,测试了caspase-3的活化作用,其浓度比对照提高了近14倍。此外,化合物的酶抑制试验针对两个常见的抗肿瘤靶标,即KDM1和CDK1评估了图4,它们显示出显着的抑制活性,IC 50分别为0.096和0.078μM 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号