Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.bioorg.2020.104050 Safura Jokar 1 , Mostafa Erfani 2 , Omid Bavi 3 , Saeedeh Khazaei 4 , Mohammad Sharifzadeh 5 , Malihe Hajiramezanali 1 , Davood Beiki 6 , Amir Shamloo 7
|
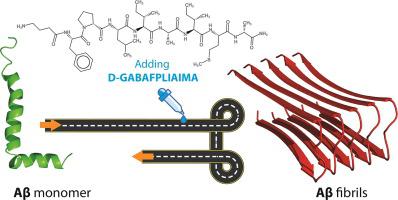
Formation of the amyloid beta (Aβ) peptide aggregations represents an indispensable role in appearing and progression of Alzheimer disease. β-sheet breaker peptides can be designed and modified with different amino acids in order to improve biological properties and binding affinity to the amyloid beta peptide. In the present study, three peptide sequences were designed based on the hopeful results of LIAIMA peptide and molecular docking studies were carried out onto the monomer and fibril structure of amyloid beta peptide using AutoDock Vina software. According to the obtained interactions and binding energy from docking, the best-designed peptide (d-GABA-FPLIAIMA) was chosen and synthesized in great yield (%96) via the Fmoc solid-phase peptide synthesis. The synthesis and purity of the resulting peptide were estimated and evaluated by Mass spectroscopy and Reversed-phase high-performance liquid chromatography (RP-HPLC) methods, respectively. Stability studies in plasma and Thioflavin T (ThT) assay were performed in order to measure the binding affinity and in vitro aggregation inhibition of Aβ peptide. The d-GABA-FPLIAIMA peptide showed good binding energy and affinity to Aβ fibrils, high stability (more than 90%) in human serum, and a reduction of 20% in inhibition of the Aβ aggregation growth. Finally, the favorable characteristics of our newly designed peptide make it a promising candidate β-sheet breaker agent for further in vivo studies.
中文翻译:

抗淀粉样蛋白β聚集的肽基抑制剂的设计:分子对接,合成和体外评估。
淀粉样β(Aβ)肽聚集体的形成代表阿尔茨海默氏病的发生和发展中不可或缺的作用。可以设计和修饰具有不同氨基酸的β-折叠阻滞肽,以改善生物学特性和与淀粉样β肽的结合亲和力。在本研究中,基于LIAIMA肽的希望结果设计了三个肽序列,并使用AutoDock Vina软件对淀粉样β肽的单体和原纤维结构进行了分子对接研究。根据对接中获得的相互作用和结合能,设计最佳的肽(d(GABA-FPLIAIMA)被选择并通过Fmoc固相肽合成以高收率(%96)合成。分别通过质谱法和反相高效液相色谱法(RP-HPLC)评估和评估所得肽的合成和纯度。为了测量Aβ肽的结合亲和力和体外聚集抑制作用,进行了血浆和硫黄素T(ThT)分析的稳定性研究。所述d -GABA-FPLIAIMA肽显示出良好的结合能和亲和力与Aβ原纤维,在人血清中的稳定性高(超过90%),以及在抑制Aβ聚集生长的减少的20%。最后,我们新设计的肽的良好特性使其成为有希望的候选β-折叠阻滞剂,可用于进一步的体内研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号