Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.apsb.2020.06.011 Kun Zhang , Yu Zhao , Zheng Zhang , Mengyao Zhang , Xiaodong Wu , Huijie Bian , Ping Zhu , Zhinan Chen
|
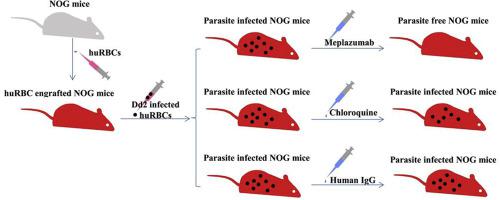
Meplazumab is an anti-CD147 humanized IgG2 antibody. The purpose of this study was to characterize the nonclinical safety, tolerance and efficacy evaluation of meplazumab treating chloroquine resistant Plasmodium falciparum. Meplazumab was well tolerated in repeat-dose toxicology studies in cynomolgus monkeys. No observed adverse effect level was 12 mg/kg. No difference between genders in the primary toxicokinetic parameters after repeat intravenous injection of meplazumab. No increased levels of drug exposure and drug accumulation were observed in different gender and dose groups. Meplazumab had a low cross-reactivity rate in various tissues and did not cause hemolysis or aggregation of red blood cells. The biodistribution and excretion results indicated that meplazumab was mainly distributed in the plasma, whole blood, and hemocytes, and excreted in the urine. Moreover, meplazumab effectively inhibited the parasites from invading erythrocytes in humanized mice in a time-dependent manner and the efficacy is superior to that of chloroquine. All these studies suggested that meplazumab is safe and well tolerated in cynomolgus monkeys, and effectively inhibits P. falciparum from invading into human red blood cells. These nonclinical data facilitated the initiation of an ongoing clinical trial of meplazumab for antimalarial therapy.
中文翻译:

美普珠单抗治疗耐氯喹的恶性疟原虫的非临床安全性,耐受性和药效学评估
Meplazumab是抗CD147人源化IgG2抗体。这项研究的目的是表征美普珠单抗治疗对氯喹耐药的恶性疟原虫的非临床安全性,耐受性和疗效评估。在食蟹猴的重复剂量毒理学研究中,对Meplazumab的耐受性良好。没有观察到不良反应水平为12 mg / kg。重复静脉注射美普珠单抗后,主要毒物动力学参数的性别无差异。在不同性别和剂量组中均未观察到药物暴露和药物蓄积的增加水平。Meplazumab在各种组织中的交叉反应率较低,并且不会引起溶血或红细胞聚集。生物分布和排泄结果表明,meplazumab主要分布在血浆,全血和血细胞中,并在尿中排泄。而且,甲普拉珠单抗以时间依赖性方式有效抑制人源化小鼠中的寄生虫侵袭红细胞,并且其功效优于氯喹。恶性疟原虫侵入人的红细胞。这些非临床数据促进了正在进行的抗疟疾药物美普珠单抗临床试验的启动。










































 京公网安备 11010802027423号
京公网安备 11010802027423号