Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Micro-strains in the extracellular matrix induce angiogenesis.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-06-30 , DOI: 10.1039/d0lc00145g Mary Kathryn Sewell-Loftin 1 , Joshua B Katz , Steven C George , Gregory D Longmore
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-06-30 , DOI: 10.1039/d0lc00145g Mary Kathryn Sewell-Loftin 1 , Joshua B Katz , Steven C George , Gregory D Longmore
Affiliation
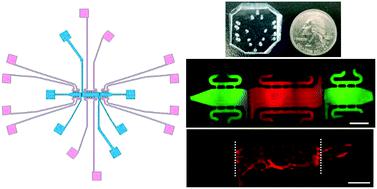
|
An improved understanding of biomechanical factors that control tumor development, including angiogenesis, could explain why few of the promising treatment strategies discovered via in vitro models translate well into in vivo or clinical studies. The ability to manipulate and in real-time study the multiple independent biomechanical properties on cellular activity has been limited, primarily due to limitations in traditional in vitro platforms or the inability to manipulate such factors in vivo. We present a novel microfluidic platform that mimics the vascularized tumor microenvironment with independent control of interstitial flow and mechanical strain. The microtissue platform design isolates mechanically-stimulated angiogenesis in the tumor microenvironment, by manipulating interstitial flow to eliminate soluble factors that could drive blood vessel growth. Our studies demonstrate that enhanced mechanical strain induced by cancer-associated fibroblasts (CAFs) promotes angiogenesis in microvasculature models, even when preventing diffusion of soluble factors to the growing vasculature. Moreover, small but significant decreases in micro-strains induced by inhibited CAFs were sufficient to reduce angiogenesis. Ultimately, we believe this platform represents a significant advancement in the ability to investigate biomechanical signals while controlling for biochemical signals, with a potential to be utilized in fields beyond cancer research.
中文翻译:

细胞外基质中的微应变诱导血管生成。
对控制肿瘤发展(包括血管生成)的生物力学因素的进一步了解可以解释为什么通过体外模型发现的有希望的治疗策略很少能很好地转化为体内或临床研究。操纵和实时研究细胞活动的多种独立生物力学特性的能力受到限制,这主要是由于传统体外平台的限制或无法在体内操纵这些因素。我们提出了一种新颖的微流体平台,可以模拟血管化的肿瘤微环境,并独立控制间质流动和机械应变。微组织平台设计通过操纵间质流动来消除可能驱动血管生长的可溶性因子,从而隔离肿瘤微环境中机械刺激的血管生成。我们的研究表明,癌症相关成纤维细胞 (CAF) 诱导的机械应变增强可促进微脉管系统模型中的血管生成,即使在阻止可溶性因子扩散到生长中的脉管系统时也是如此。此外,受抑制的 CAF 诱导的微应变小幅但显着的降低足以减少血管生成。最终,我们相信该平台代表了在控制生化信号的同时研究生物力学信号的能力方面的重大进步,并有可能应用于癌症研究以外的领域。
更新日期:2020-07-29
中文翻译:

细胞外基质中的微应变诱导血管生成。
对控制肿瘤发展(包括血管生成)的生物力学因素的进一步了解可以解释为什么通过体外模型发现的有希望的治疗策略很少能很好地转化为体内或临床研究。操纵和实时研究细胞活动的多种独立生物力学特性的能力受到限制,这主要是由于传统体外平台的限制或无法在体内操纵这些因素。我们提出了一种新颖的微流体平台,可以模拟血管化的肿瘤微环境,并独立控制间质流动和机械应变。微组织平台设计通过操纵间质流动来消除可能驱动血管生长的可溶性因子,从而隔离肿瘤微环境中机械刺激的血管生成。我们的研究表明,癌症相关成纤维细胞 (CAF) 诱导的机械应变增强可促进微脉管系统模型中的血管生成,即使在阻止可溶性因子扩散到生长中的脉管系统时也是如此。此外,受抑制的 CAF 诱导的微应变小幅但显着的降低足以减少血管生成。最终,我们相信该平台代表了在控制生化信号的同时研究生物力学信号的能力方面的重大进步,并有可能应用于癌症研究以外的领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号