当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Benzanilide Crystals in Organic Solvents
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jced.0c00167 Xiao Juan Liu 1 , Yang Zhang 1 , Xue Zhong Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jced.0c00167 Xiao Juan Liu 1 , Yang Zhang 1 , Xue Zhong Wang 1, 2
Affiliation
|
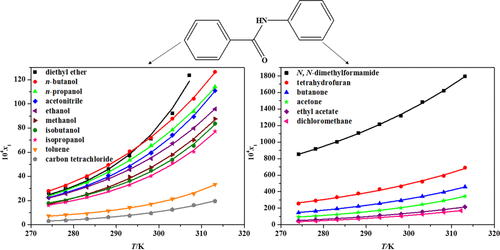
The solubility of benzanilide in sixteen pure organic solvents, including N, N-dimethylformamide, tetrahydrofuran, butanone, acetone, ethyl acetate, dichloromethane, n-butanol, diethyl ether, n-propanol, acetonitrile, ethanol, methanol, isobutanol, isopropanol, toluene, and carbon tetrachloride, was measured at temperatures ranging from 274.15 to 313.15 K using the static equilibrium method at atmospheric pressure. The modified Apelblat model, Buchowski-Ksiazczak λh model, and Non Random Two Liquid (NRTL) model were used to correlate the experimental solubility data. It was found that in all these solvents the solubility of benzanilide increases in different pace as the temperature increases. The modified Apelblat model gave the best fit. Guided by the solubility data, preliminary experimental study was conducted for cooling crystallization of benzanilide in ethanol and n-propanol. The result indicated that the solvent, stirring speed and cooling rate all have significant impact on crystal morphology and particle size distribution.
中文翻译:

苯甲酰苯胺晶体在有机溶剂中的溶解度
苯甲酰苯胺在16种纯有机溶剂中的溶解度,包括N,N-二甲基甲酰胺,四氢呋喃,丁酮,丙酮,乙酸乙酯,二氯甲烷,正丁醇,乙醚,正丙醇,乙腈,乙醇,甲醇,异丁醇,异丙醇,甲苯使用大气压下的静态平衡法在274.15至313.15 K的温度范围内测量了四氯化碳和四氯化碳。修正的Apelblat模型Buchowski- Ksiazczakλh模型和非随机两种液体(NRTL)模型用于关联实验溶解度数据。发现在所有这些溶剂中,苯甲酰苯胺的溶解度随着温度的升高而以不同的速度增加。修改后的Apelblat模型提供了最佳拟合。在溶解度数据的指导下,进行了对苯甲酰苯胺在乙醇和正丙醇中冷却结晶的初步实验研究。结果表明,溶剂,搅拌速度和冷却速率均对晶体形态和粒径分布有显着影响。
更新日期:2020-08-14
中文翻译:

苯甲酰苯胺晶体在有机溶剂中的溶解度
苯甲酰苯胺在16种纯有机溶剂中的溶解度,包括N,N-二甲基甲酰胺,四氢呋喃,丁酮,丙酮,乙酸乙酯,二氯甲烷,正丁醇,乙醚,正丙醇,乙腈,乙醇,甲醇,异丁醇,异丙醇,甲苯使用大气压下的静态平衡法在274.15至313.15 K的温度范围内测量了四氯化碳和四氯化碳。修正的Apelblat模型Buchowski- Ksiazczakλh模型和非随机两种液体(NRTL)模型用于关联实验溶解度数据。发现在所有这些溶剂中,苯甲酰苯胺的溶解度随着温度的升高而以不同的速度增加。修改后的Apelblat模型提供了最佳拟合。在溶解度数据的指导下,进行了对苯甲酰苯胺在乙醇和正丙醇中冷却结晶的初步实验研究。结果表明,溶剂,搅拌速度和冷却速率均对晶体形态和粒径分布有显着影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号