当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Organophosphorous Pesticide Fosthiazate: Absolute Configuration, Stereoselective Bioactivity, Toxicity, and Degradation in Vegetables.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.jafc.0c03008 Lianshan Li 1 , Jiangyan Xu 2 , Bo Lv 2 , Amir E Kaziem 1 , Fang Liu 2 , Haiyan Shi 1 , Minghua Wang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.jafc.0c03008 Lianshan Li 1 , Jiangyan Xu 2 , Bo Lv 2 , Amir E Kaziem 1 , Fang Liu 2 , Haiyan Shi 1 , Minghua Wang 1
Affiliation
|
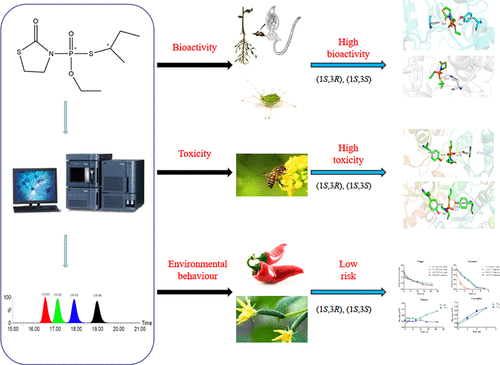
Fosthiazate is a widely used chiral organophosphorous nematicide with four stereoisomers. The present study systemically assessed the stereoselectivity of fosthiazate for the first time, including absolute configuration confirmation, stereoselective bioactivity toward nematode and aphid, toxicity to honeybees, and stereoselective degradation in cucumber and pepper under field conditions. The absolute configurations of the four stereoisomers that eluted on the Superchiral IG-3 column were confirmed as (1S,3R)-(−)-fosthiazate, (1S,3S)-(−)-fosthiazate, (1R,3S)-(+)-fosthiazate, and (1R,3R)-(+)-fosthiazate. In comparison to the other two stereoisomers, (1S,3R)-fosthiazate and (1S,3S)-fosthiazate possess more than 100 times bioactivity and 10 times toxicity toward the target and non-target organisms, respectively. The molecular docking found that (1S,3R)-fosthiazate and (1S,3S)-fosthiazate had shorter binding distances and lower energies with acetylcholinesterase (AChE), which illuminated the mechanism of the experimental results. In addition, both of the high-bioactive stereoisomers had faster degradation rates in cucumber and pepper. On the basis of the results of bioactivity, toxicity, and degradation behavior, the stereoisomer mixture with (1S,3R)-fosthiazate and (1S,3S)-fosthiazate will be a better option than racemic fosthiazate to increase the bioactivity and reduce application rates.
中文翻译:

手性有机磷农药邻苯二甲酸酯:绝对构型,立体选择性生物活性,毒性和蔬菜中的降解。
Fosthiazate是一种广泛使用的具有四个立体异构体的手性有机磷杀线虫剂。本研究首次系统地评估了邻苯二甲酸酯的立体选择性,包括绝对构型确认,对线虫和蚜虫的立体选择性生物活性,对蜜蜂的毒性以及田间条件下黄瓜和胡椒的立体选择性降解。证实在Superchiral IG-3色谱柱上洗脱的四种立体异构体的绝对构型为(1 S,3 R)-(-)-邻硫杂酸酯,(1 S,3 S)-(-)-邻硫杂酸酯,(1 R,3 S)-(+)-邻硫杂酸酯和(1 R,3 R)-(+)-邻苯二甲酸酯。与其他两种立体异构体相比,(1 S,3 R)-邻苯二酸酯和(1 S,3 S)-邻苯二酸酯分别对目标生物和非目标生物具有100倍以上的生物活性和10倍的毒性。分子对接发现(1 S,3 R)-噻唑酸盐和(1 S,3 S-甲硫唑酸盐与乙酰胆碱酯酶(AChE)的结合距离更短,能量更低,这说明了实验结果的机理。另外,两种高生物活性立体异构体在黄瓜和辣椒中的降解速度都更快。根据生物活性,毒性和降解行为的结果,具有(1 S,3 R)-邻苯二甲酸酯和(1 S,3 S)-邻苯二甲酸酯的立体异构体混合物比外消旋的邻苯二甲酸酯具有更好的生物活性。并降低使用率。
更新日期:2020-07-22
中文翻译:

手性有机磷农药邻苯二甲酸酯:绝对构型,立体选择性生物活性,毒性和蔬菜中的降解。
Fosthiazate是一种广泛使用的具有四个立体异构体的手性有机磷杀线虫剂。本研究首次系统地评估了邻苯二甲酸酯的立体选择性,包括绝对构型确认,对线虫和蚜虫的立体选择性生物活性,对蜜蜂的毒性以及田间条件下黄瓜和胡椒的立体选择性降解。证实在Superchiral IG-3色谱柱上洗脱的四种立体异构体的绝对构型为(1 S,3 R)-(-)-邻硫杂酸酯,(1 S,3 S)-(-)-邻硫杂酸酯,(1 R,3 S)-(+)-邻硫杂酸酯和(1 R,3 R)-(+)-邻苯二甲酸酯。与其他两种立体异构体相比,(1 S,3 R)-邻苯二酸酯和(1 S,3 S)-邻苯二酸酯分别对目标生物和非目标生物具有100倍以上的生物活性和10倍的毒性。分子对接发现(1 S,3 R)-噻唑酸盐和(1 S,3 S-甲硫唑酸盐与乙酰胆碱酯酶(AChE)的结合距离更短,能量更低,这说明了实验结果的机理。另外,两种高生物活性立体异构体在黄瓜和辣椒中的降解速度都更快。根据生物活性,毒性和降解行为的结果,具有(1 S,3 R)-邻苯二甲酸酯和(1 S,3 S)-邻苯二甲酸酯的立体异构体混合物比外消旋的邻苯二甲酸酯具有更好的生物活性。并降低使用率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号