当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia-Mediated Bromate Inhibition during Ozonation Promotes the Toxicity Due to Organic Byproduct Transformation.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.est.0c02984 Qian-Yuan Wu 1 , Lu-Lin Yang 1 , Xin-Yang Zhang 1 , Wen-Long Wang 2 , Yao Lu 3 , Ye Du 3 , Yun Lu 2 , Hong-Ying Hu 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.est.0c02984 Qian-Yuan Wu 1 , Lu-Lin Yang 1 , Xin-Yang Zhang 1 , Wen-Long Wang 2 , Yao Lu 3 , Ye Du 3 , Yun Lu 2 , Hong-Ying Hu 2, 3
Affiliation
|
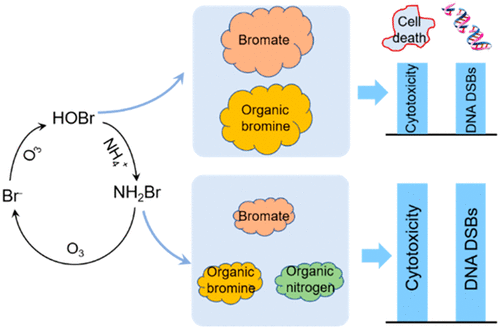
Ammonia (NH4+) and hydrogen peroxide (H2O2) have been widely used to inhibit bromate formation during ozonation. However, organic byproducts can also pose a risk under these conditions. During bromate inhibition, the influence of NH4+ and H2O2 on organic byproducts and their toxicity should be elucidated. Our study found that NH4+ suppressed organic bromine, but might result in increased toxicity. Adding 0.5 mg/L of NH4+–N substantially increased both the formation of cytotoxicity and genotoxicity (DNA double-strand breaks) of organic byproducts from 0.6 to 1.6 mg-phenol/L, and from 0.3 to 0.8 μg-4-NQO/L (0.5 mg/L Br–, 5 mg/L O3). NH4+ decreased bromate, but increased the overall toxicity of the integrated byproducts (organic byproducts and bromate). Organic nitrogen measurements and 15N isotope analysis showed enhanced incorporation of nitrogen into organic matter when NH4+ and Br– coexisted during ozonation. NH4+ decreased the formation of brominated acetonitriles, but enhanced the formation of brominated nitromethanes and brominated acetamides. These brominated nitrogenous byproducts were partially responsible for this increase in toxicity. Different from ammonia, H2O2 could reduce both bromate and the toxicity of organic byproducts. In the presence of 0.5 mg/L Br– and 10 mg/L O3, adding H2O2 (0.5 mM) substantially suppressed bromate, cytotoxicity formation and genotoxicity formation by 88%, 63% and 67%. This study highlights that focusing on bromate control with NH4+ addition might result in higher toxicity. Efforts are needed to effectively control the toxicities of bromate and organic byproducts simultaneously.
中文翻译:

在臭氧化过程中,氨介导的溴酸盐抑制作用会由于有机副产物转化而促进毒性。
氨(NH 4 +)和过氧化氢(H 2 O 2)已被广泛用于抑制臭氧化过程中溴酸盐的形成。但是,有机副产物在这些条件下也可能带来风险。在溴酸盐抑制过程中,应阐明NH 4 +和H 2 O 2对有机副产物及其毒性的影响。我们的研究发现NH 4 +抑制了有机溴,但可能导致毒性增加。添加0.5 mg / L NH 4 +–N大大增加了有机副产物的细胞毒性和遗传毒性(DNA双链断裂)的形成,从0.6到1.6 mg-酚/ L,从0.3到0.8μg-4-NQO/ L(0.5 mg / L Br –,5 mg / LO 3)。NH 4 +减少了溴酸盐,但增加了综合副产物(有机副产物和溴酸盐)的总体毒性。有机氮的测量和15个Ñ同位素分析表现出增强的氮引入到有机物质时NH 4 +和Br -臭氧化过程中共存。NH 4 +减少了溴化乙腈的形成,但增加了溴化硝基甲烷和溴化乙酰胺的形成。这些溴化的含氮副产物是造成这种毒性增加的部分原因。与氨不同,H 2 O 2可同时降低溴酸盐和有机副产物的毒性。在0.5 mg / L的溴的存在下-和10mg / LO 3,加入ħ 2 ö 2由88%,63%和67%(0.5毫摩尔)基本上抑制溴酸盐,细胞毒性的形成和遗传毒性的形成。这项研究强调,着重于用NH 4 +控制溴酸盐添加可能会导致更高的毒性。需要努力有效地同时控制溴酸盐和有机副产物的毒性。
更新日期:2020-07-21
中文翻译:

在臭氧化过程中,氨介导的溴酸盐抑制作用会由于有机副产物转化而促进毒性。
氨(NH 4 +)和过氧化氢(H 2 O 2)已被广泛用于抑制臭氧化过程中溴酸盐的形成。但是,有机副产物在这些条件下也可能带来风险。在溴酸盐抑制过程中,应阐明NH 4 +和H 2 O 2对有机副产物及其毒性的影响。我们的研究发现NH 4 +抑制了有机溴,但可能导致毒性增加。添加0.5 mg / L NH 4 +–N大大增加了有机副产物的细胞毒性和遗传毒性(DNA双链断裂)的形成,从0.6到1.6 mg-酚/ L,从0.3到0.8μg-4-NQO/ L(0.5 mg / L Br –,5 mg / LO 3)。NH 4 +减少了溴酸盐,但增加了综合副产物(有机副产物和溴酸盐)的总体毒性。有机氮的测量和15个Ñ同位素分析表现出增强的氮引入到有机物质时NH 4 +和Br -臭氧化过程中共存。NH 4 +减少了溴化乙腈的形成,但增加了溴化硝基甲烷和溴化乙酰胺的形成。这些溴化的含氮副产物是造成这种毒性增加的部分原因。与氨不同,H 2 O 2可同时降低溴酸盐和有机副产物的毒性。在0.5 mg / L的溴的存在下-和10mg / LO 3,加入ħ 2 ö 2由88%,63%和67%(0.5毫摩尔)基本上抑制溴酸盐,细胞毒性的形成和遗传毒性的形成。这项研究强调,着重于用NH 4 +控制溴酸盐添加可能会导致更高的毒性。需要努力有效地同时控制溴酸盐和有机副产物的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号