当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Parameters at Bio-Nano Interface and Nanomaterial Toxicity: A Case Study on BSA Interaction with ZnO, SiO2, and TiO2.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.chemrestox.9b00468 Aurica Precupas 1 , Daniela Gheorghe 1 , Alina Botea-Petcu 1 , Anca Ruxandra Leonties 1 , Romica Sandu 1 , Vlad Tudor Popa 1 , Espen Mariussen 2 , El Yamani Naouale 2 , Elise Rundén-Pran 2 , Veronica Dumit 3 , Ying Xue 4 , Mihaela Roxana Cimpan 4 , Maria Dusinska 2 , Andrea Haase 3 , Speranta Tanasescu 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-06-29 , DOI: 10.1021/acs.chemrestox.9b00468 Aurica Precupas 1 , Daniela Gheorghe 1 , Alina Botea-Petcu 1 , Anca Ruxandra Leonties 1 , Romica Sandu 1 , Vlad Tudor Popa 1 , Espen Mariussen 2 , El Yamani Naouale 2 , Elise Rundén-Pran 2 , Veronica Dumit 3 , Ying Xue 4 , Mihaela Roxana Cimpan 4 , Maria Dusinska 2 , Andrea Haase 3 , Speranta Tanasescu 1
Affiliation
|
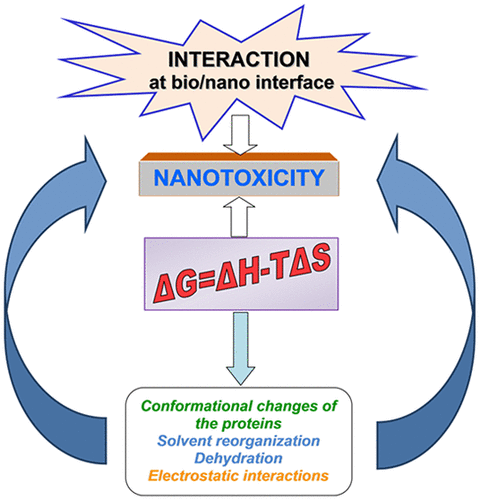
Understanding nanomaterial (NM)–protein interactions is a key issue in defining the bioreactivity of NMs with great impact for nanosafety. In the present work, the complex phenomena occurring at the bio/nano interface were evaluated in a simple case study focusing on NM–protein binding thermodynamics and protein stability for three representative metal oxide NMs, namely, zinc oxide (ZnO; NM-110), titanium dioxide (TiO2; NM-101), and silica (SiO2; NM-203). The thermodynamic signature associated with the NM interaction with an abundant protein occurring in most cell culture media, bovine serum albumin (BSA), has been investigated by isothermal titration and differential scanning calorimetry. Circular dichroism spectroscopy offers additional information concerning adsorption-induced protein conformational changes. The BSA adsorption onto NMs is enthalpy-controlled, with the enthalpic character (favorable interaction) decreasing as follows: ZnO (NM-110) > SiO2 (NM-203) > TiO2 (NM-101). The binding of BSA is spontaneous, as revealed by the negative free energy, ΔG, for all systems. The structural stability of the protein decreased as follows: TiO2 (NM-101) > SiO2 (NM-203) > ZnO (NM-110). As protein binding may alter NM reactivity and thus the toxicity, we furthermore assessed its putative influence on DNA damage, as well as on the expression of target genes for cell death (RIPK1, FAS) and oxidative stress (SOD1, SOD2, CAT, GSTK1) in the A549 human alveolar basal epithelial cell line. The enthalpic component of the BSA–NM interaction, corroborated with BSA structural stability, matched the ranking for the biological alterations, i.e., DNA strand breaks, oxidized DNA lesions, cell-death, and antioxidant gene expression in A549 cells. The relative and total content of BSA in the protein corona was determined using mass-spectrometry-based proteomics. For the present case study, the thermodynamic parameters at bio/nano interface emerge as key descriptors for the dominant contributions determining the adsorption processes and NMs toxicological effect.
中文翻译:

生物纳米界面的热力学参数和纳米材料毒性:BSA 与 ZnO、SiO2 和 TiO2 相互作用的案例研究。
了解纳米材料 (NM)-蛋白质相互作用是定义纳米材料生物反应性的关键问题,对纳米安全有重大影响。在目前的工作中,在一个简单的案例研究中评估了发生在生物/纳米界面的复杂现象,重点是三种代表性金属氧化物 NM,即氧化锌(ZnO;NM-110)的 NM-蛋白质结合热力学和蛋白质稳定性、二氧化钛 (TiO 2 ; NM-101) 和二氧化硅 (SiO 2; NM-203)。通过等温滴定和差示扫描量热法研究了与 NM 与大多数细胞培养基中存在的丰富蛋白质、牛血清白蛋白 (BSA) 相互作用相关的热力学特征。圆二色光谱提供了有关吸附诱导的蛋白质构象变化的额外信息。BSA 在 NMs 上的吸附受焓控制,焓特性(有利的相互作用)如下降低:ZnO (NM-110) > SiO 2 (NM-203) > TiO 2 (NM-101)。BSA 的结合是自发的,正如所有系统的负自由能 Δ G所揭示的那样。蛋白质的结构稳定性降低如下:TiO 2 (NM-101) > SiO 2(NM-203) > 氧化锌 (NM-110)。由于蛋白质结合可能会改变 NM 反应性,从而改变毒性,我们进一步评估了其对 DNA 损伤以及细胞死亡(RIPK1、FAS)和氧化应激(SOD1、SOD2、CAT、GSTK1)靶基因表达的推定影响) 在 A549 人肺泡基底上皮细胞系中。BSA-NM 相互作用的焓分量与 BSA 结构稳定性相一致,与生物学改变的排名相匹配,即 A549 细胞中的 DNA 链断裂、氧化的 DNA 损伤、细胞死亡和抗氧化基因表达。使用基于质谱的蛋白质组学确定蛋白质电晕中 BSA 的相对和总含量。对于目前的案例研究,
更新日期:2020-08-17
中文翻译:

生物纳米界面的热力学参数和纳米材料毒性:BSA 与 ZnO、SiO2 和 TiO2 相互作用的案例研究。
了解纳米材料 (NM)-蛋白质相互作用是定义纳米材料生物反应性的关键问题,对纳米安全有重大影响。在目前的工作中,在一个简单的案例研究中评估了发生在生物/纳米界面的复杂现象,重点是三种代表性金属氧化物 NM,即氧化锌(ZnO;NM-110)的 NM-蛋白质结合热力学和蛋白质稳定性、二氧化钛 (TiO 2 ; NM-101) 和二氧化硅 (SiO 2; NM-203)。通过等温滴定和差示扫描量热法研究了与 NM 与大多数细胞培养基中存在的丰富蛋白质、牛血清白蛋白 (BSA) 相互作用相关的热力学特征。圆二色光谱提供了有关吸附诱导的蛋白质构象变化的额外信息。BSA 在 NMs 上的吸附受焓控制,焓特性(有利的相互作用)如下降低:ZnO (NM-110) > SiO 2 (NM-203) > TiO 2 (NM-101)。BSA 的结合是自发的,正如所有系统的负自由能 Δ G所揭示的那样。蛋白质的结构稳定性降低如下:TiO 2 (NM-101) > SiO 2(NM-203) > 氧化锌 (NM-110)。由于蛋白质结合可能会改变 NM 反应性,从而改变毒性,我们进一步评估了其对 DNA 损伤以及细胞死亡(RIPK1、FAS)和氧化应激(SOD1、SOD2、CAT、GSTK1)靶基因表达的推定影响) 在 A549 人肺泡基底上皮细胞系中。BSA-NM 相互作用的焓分量与 BSA 结构稳定性相一致,与生物学改变的排名相匹配,即 A549 细胞中的 DNA 链断裂、氧化的 DNA 损伤、细胞死亡和抗氧化基因表达。使用基于质谱的蛋白质组学确定蛋白质电晕中 BSA 的相对和总含量。对于目前的案例研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号