当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Modifications Critical for Myonectin/Erythroferrone Secretion and Oligomer Assembly.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.biochem.0c00461 Ashley N Stewart 1 , Hannah C Little 1 , David J Clark 2 , Hui Zhang 2 , G William Wong 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.biochem.0c00461 Ashley N Stewart 1 , Hannah C Little 1 , David J Clark 2 , Hui Zhang 2 , G William Wong 1
Affiliation
|
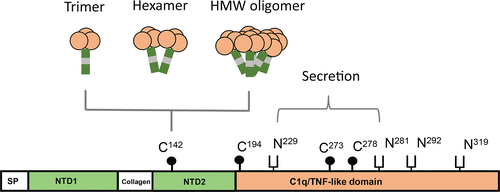
Myonectin/erythroferrone (also known as CTRP15) is a secreted hormone with metabolic function and a role in stress erythropoiesis. Despite its importance in physiologic processes, biochemical characterization of the protein is lacking. Here, we show that multiple protein modifications are critical for myonectin secretion and multimerization. Abolishing N-linked glycosylation by tunicamycin, glucosamine supplementation, or glutamine substitutions of all four potential Asn glycosylation sites blocked myonectin secretion. Mass spectrometry confirmed that Asn-229 and Asn-281 were glycosylated, and substituting both Asn sites with Gln prevented myonectin secretion. Although Asn-319 is not identified as glycosylated, Gln substitution caused protein misfolding and retention in the endoplasmic reticulum. Of the four conserved cysteines, Cys-273 and Cys-278 were required for proper protein folding; Ala substitution of either site inhibited protein secretion. In contrast, Ala substitutions of Cys-142, Cys-194, or both markedly enhanced protein secretion, suggesting endoplasmic reticulum retention that facilitates myonectin oligomer assembly. Secreted myonectin consists of trimers, hexamers, and high-molecular weight (HMW) oligomers. The formation of higher-order structures via intermolecular disulfide bonds depended on Cys-142 and Cys-194; while the C142A mutant formed almost exclusively trimers, the C194A mutant was impaired in HMW oligomer formation. Most Pro residues within the short collagen domain of myonectin were also hydroxylated, a modification that stabilized the collagen triple helix. Inhibiting Pro hydroxylation or deleting the collagen domain markedly reduced the rate of protein secretion. Together, our results reveal key determinants that are important for myonectin folding, secretion, and multimeric assembly and provide a basis for future structure–function studies.
中文翻译:

蛋白质修饰对于Myonectin / Eurthroferrone分泌和寡聚体组装至关重要。
Myonectin / eryferroferrone(也称为CTRP15)是一种分泌的激素,具有代谢功能,并在应激性红细胞生成中起作用。尽管其在生理过程中很重要,但缺乏蛋白质的生化特性。在这里,我们显示了多种蛋白质修饰对于Myonectin分泌和多聚化至关重要。通过衣霉素,葡萄糖胺补充或全部四个潜在的Asn糖基化位点的谷氨酰胺取代来消除N-联糖基化,从而阻止了肌凝素的分泌。质谱法证实Asn-229和Asn-281被糖基化,并且用Gln取代两个Asn位点可以阻止粘连蛋白的分泌。尽管Asn-319未鉴定为糖基化的,但Gln取代导致蛋白质错误折叠并保留在内质网中。在四个保守的半胱氨酸中,需要Cys-273和Cys-278才能正确折叠蛋白质。任一位点的丙氨酸取代均可抑制蛋白质分泌。相反,Cys-142,Cys-194或两者的Ala取代显着增强了蛋白质分泌,表明内质网的保留有利于Myonectin寡聚物的组装。分泌的粘连蛋白由三聚体,六聚体和高分子量(HMW)低聚物组成。通过分子间二硫键形成高阶结构取决于Cys-142和Cys-194。虽然C142A突变体几乎只形成三聚体,但C194A突变体在HMW低聚物形成中受损。粘连蛋白的短胶原结构域内的大多数Pro残基也被羟基化,这种修饰可稳定胶原三螺旋。抑制Pro羟基化或删除胶原蛋白结构域可显着降低蛋白质分泌速率。在一起,我们的结果揭示了关键的决定因素,这些决定因素对于Myonectin的折叠,分泌和多聚体组装很重要,并为将来的结构功能研究提供了基础。
更新日期:2020-07-28
中文翻译:

蛋白质修饰对于Myonectin / Eurthroferrone分泌和寡聚体组装至关重要。
Myonectin / eryferroferrone(也称为CTRP15)是一种分泌的激素,具有代谢功能,并在应激性红细胞生成中起作用。尽管其在生理过程中很重要,但缺乏蛋白质的生化特性。在这里,我们显示了多种蛋白质修饰对于Myonectin分泌和多聚化至关重要。通过衣霉素,葡萄糖胺补充或全部四个潜在的Asn糖基化位点的谷氨酰胺取代来消除N-联糖基化,从而阻止了肌凝素的分泌。质谱法证实Asn-229和Asn-281被糖基化,并且用Gln取代两个Asn位点可以阻止粘连蛋白的分泌。尽管Asn-319未鉴定为糖基化的,但Gln取代导致蛋白质错误折叠并保留在内质网中。在四个保守的半胱氨酸中,需要Cys-273和Cys-278才能正确折叠蛋白质。任一位点的丙氨酸取代均可抑制蛋白质分泌。相反,Cys-142,Cys-194或两者的Ala取代显着增强了蛋白质分泌,表明内质网的保留有利于Myonectin寡聚物的组装。分泌的粘连蛋白由三聚体,六聚体和高分子量(HMW)低聚物组成。通过分子间二硫键形成高阶结构取决于Cys-142和Cys-194。虽然C142A突变体几乎只形成三聚体,但C194A突变体在HMW低聚物形成中受损。粘连蛋白的短胶原结构域内的大多数Pro残基也被羟基化,这种修饰可稳定胶原三螺旋。抑制Pro羟基化或删除胶原蛋白结构域可显着降低蛋白质分泌速率。在一起,我们的结果揭示了关键的决定因素,这些决定因素对于Myonectin的折叠,分泌和多聚体组装很重要,并为将来的结构功能研究提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号