当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Characterization of Sphingomonas sp. KT-1 PahZ1-Catalyzed Biodegradation of Thermally Synthesized Poly(aspartic acid)
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-06-30 , DOI: 10.1021/acssuschemeng.0c01158 Chad A. Brambley 1 , Austin L. Bolay 2 , Henry Salvo 3 , Amanda L. Jansch 2 , Tarah J. Yared 2 , Justin M. Miller 1 , Jamie R. Wallen 3 , Mitch H. Weiland 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-06-30 , DOI: 10.1021/acssuschemeng.0c01158 Chad A. Brambley 1 , Austin L. Bolay 2 , Henry Salvo 3 , Amanda L. Jansch 2 , Tarah J. Yared 2 , Justin M. Miller 1 , Jamie R. Wallen 3 , Mitch H. Weiland 2
Affiliation
|
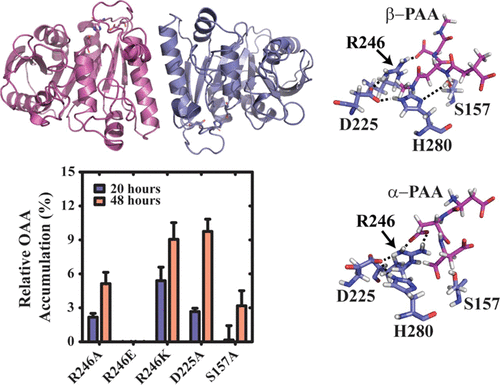
Poly(aspartic acid) (PAA) is a green alternative to non-biodegradable poly(carboxylates) and has applications in both industrial and biomedical settings. PAA is synthesized by heating monomeric aspartic acid to yield a polysuccinamide that can be ring-opened to yield thermal PAA composed of 30% α-amide and 70% β-amide linkages. Here, we report the first X-ray crystal structure of a PAA hydrolase from the bacteria Sphingomonas sp. KT-1 (PahZ1KT-1) which functions to degrade synthetic PAA to oligo(aspartic acid) by selective cleavage of β-amide linkages. The structure was solved to 2.45 Å and shows a dimeric assembly where each monomer maintains an α/β hydrolase fold with a prominent, positively lined trough responsible for binding the anionic polymeric substrate. The putative catalytic sites of each monomer lie at the surface of the enzyme on opposite faces. The dimeric interface, as supported by small-angle X-ray scattering/multi-angle light scattering data, is primarily hydrophobic and is further stabilized by flanking hydrogen bonds. Molecular dynamics simulations support the previously determined specific cleavage of only the β-amide linkage through a conformational change that aligns the substrate with the active site Ser. These data provide a scaffold for further understanding the mechanism of PAA hydrolysis and opens the opportunity for using protein engineering to catalyze the biodegradation of other xenobiotics.
中文翻译:

鞘氨醇单胞菌sp。的结构表征。KT-1 PahZ1催化热降解聚天冬氨酸的生物降解
聚天冬氨酸(PAA)是不可生物降解的聚羧酸盐的绿色替代品,在工业和生物医学领域都有应用。通过加热单体天冬氨酸以产生聚琥珀酰胺来合成PAA,该聚琥珀酰胺可以开环以产生由30%α-酰胺和70%β-酰胺键组成的热PAA。在这里,我们报告从细菌鞘氨醇单胞菌sp。PAA水解酶的第一个X射线晶体结构。KT-1(PahZ1 KT-1),可通过选择性裂解β-酰胺键将合成的PAA降解为低聚天冬氨酸。该结构解析为2.45Å,并显示出二聚体组装状态,其中每个单体保持一个α/β水解酶折叠,并带有一个明显的,带正线的低谷,负责结合阴离子聚合物基质。每个单体的推定催化位点位于酶表面的相反面上。小角度X射线散射/多角度光散射数据支持的二聚体界面主要是疏水性的,并通过侧翼氢键进一步稳定。分子动力学模拟通过将底物与活性位点Ser对齐的构象变化支持先前确定的仅β-酰胺键的特异性裂解。
更新日期:2020-07-27
中文翻译:

鞘氨醇单胞菌sp。的结构表征。KT-1 PahZ1催化热降解聚天冬氨酸的生物降解
聚天冬氨酸(PAA)是不可生物降解的聚羧酸盐的绿色替代品,在工业和生物医学领域都有应用。通过加热单体天冬氨酸以产生聚琥珀酰胺来合成PAA,该聚琥珀酰胺可以开环以产生由30%α-酰胺和70%β-酰胺键组成的热PAA。在这里,我们报告从细菌鞘氨醇单胞菌sp。PAA水解酶的第一个X射线晶体结构。KT-1(PahZ1 KT-1),可通过选择性裂解β-酰胺键将合成的PAA降解为低聚天冬氨酸。该结构解析为2.45Å,并显示出二聚体组装状态,其中每个单体保持一个α/β水解酶折叠,并带有一个明显的,带正线的低谷,负责结合阴离子聚合物基质。每个单体的推定催化位点位于酶表面的相反面上。小角度X射线散射/多角度光散射数据支持的二聚体界面主要是疏水性的,并通过侧翼氢键进一步稳定。分子动力学模拟通过将底物与活性位点Ser对齐的构象变化支持先前确定的仅β-酰胺键的特异性裂解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号