当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Isatin Thiosemicarbazone Derivatives as Potent Inhibitors of β-Amyloid Peptide Aggregation and Toxicity.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-06-29 , DOI: 10.1021/acschemneuro.0c00208 Marina Sagnou 1 , Barbara Mavroidi 1 , Archontia Kaminari 1 , Nikos Boukos 2 , Maria Pelecanou 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-06-29 , DOI: 10.1021/acschemneuro.0c00208 Marina Sagnou 1 , Barbara Mavroidi 1 , Archontia Kaminari 1 , Nikos Boukos 2 , Maria Pelecanou 1
Affiliation
|
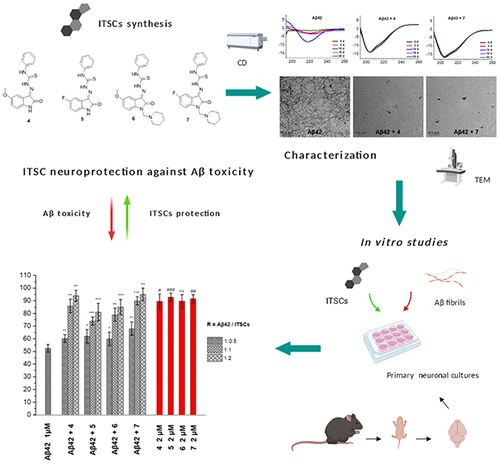
Inhibition of β-amyloid peptide (Αβ) aggregation in Alzheimer’s disease (AD) is among the therapeutic approaches against AD which still attracts scientific research interest. In the search for compounds that interact with Aβ and disrupt its typical aggregation course toward oligomeric or polymeric toxic assemblies, small organic molecules of natural origin, combining low molecular weight (necessary blood-brain barrier penetration) and low toxicity (necessary for pharmacological application), are greatly sought after. Isatin (1H-indoline-2,3-dione), a natural endogenous indole, and many of its derivatives exhibit a wide spectrum of neuropharmacological and chemotherapeutic properties. The synthesis and biological evaluation of four new isatins as inhibitors of Aβ aggregation is presented herein. In these derivatives, the N-phenyl thiosemicarbazide moiety is joined at the 3-oxo position of isatin through Schiff base formation, and substitutions are present at the indole nitrogen and position 5 of the isatin core. Biophysical studies employing circular dichroism, thioflavin T fluorescence assay, and transmission electron microscopy reveal the potential of the isatin thiosemicarbazones (ITSCs) to alter the course of Αβ aggregation, with two of the derivatives exhibiting outstanding inhibition of the aggregation process, preventing completely the formation of amyloid fibrils. Furthermore, in in vitro studies in primary neuronal cell cultures, the ITSCs were found to inhibit the Aβ-induced neurotoxicity and reactive oxygen species production at concentrations as low as 1 μM. Taken all together, the novel ITSCs can be considered as privileged structures for further development as potential AD therapeutics.
中文翻译:

新型Isatin硫杂氨基脲类衍生物作为β-淀粉样肽聚集和毒性的有效抑制剂。
在阿尔茨海默氏病(AD)中抑制β-淀粉样肽(Aβ)的聚集是针对AD的治疗方法之一,仍然吸引着科学研究的兴趣。在寻找与Aβ相互作用并破坏其典型的聚集过程的低聚或聚合毒性组合物的化合物时,天然来源的有机小分子结合了低分子量(必要的血脑屏障渗透性)和低毒性(药理学应用所需) ,倍受追捧。靛红(1个ħ-indoline-2,3-dione),一种天然的内源性吲哚及其许多衍生物具有广泛的神经药理学和化学治疗性质。本文介绍了四种新的靛红作为Aβ聚集抑制剂的合成和生物学评估。在这些衍生物中,N-苯基硫代氨基脲部分通过席夫碱的形成而连接在isatin的3-oxo位置,并且在吲哚氮和isatin核心的5位存在取代基。使用圆二色性,硫代黄素T荧光测定和透射电镜的生物物理研究表明,异丁二硫代氨基脲化合物(ITSC)可能改变Aβ聚集的过程,其中两种衍生物表现出显着的抑制聚集过程的能力,从而完全阻止了形成淀粉样原纤维。此外,在体外在原代神经元细胞培养的研究中,发现ITSC在低至1μM的浓度下抑制Aβ诱导的神经毒性和活性氧的产生。综上所述,可以将新型ITSC视为潜在的AD疗法,作为进一步发展的特权结构。
更新日期:2020-08-05
中文翻译:

新型Isatin硫杂氨基脲类衍生物作为β-淀粉样肽聚集和毒性的有效抑制剂。
在阿尔茨海默氏病(AD)中抑制β-淀粉样肽(Aβ)的聚集是针对AD的治疗方法之一,仍然吸引着科学研究的兴趣。在寻找与Aβ相互作用并破坏其典型的聚集过程的低聚或聚合毒性组合物的化合物时,天然来源的有机小分子结合了低分子量(必要的血脑屏障渗透性)和低毒性(药理学应用所需) ,倍受追捧。靛红(1个ħ-indoline-2,3-dione),一种天然的内源性吲哚及其许多衍生物具有广泛的神经药理学和化学治疗性质。本文介绍了四种新的靛红作为Aβ聚集抑制剂的合成和生物学评估。在这些衍生物中,N-苯基硫代氨基脲部分通过席夫碱的形成而连接在isatin的3-oxo位置,并且在吲哚氮和isatin核心的5位存在取代基。使用圆二色性,硫代黄素T荧光测定和透射电镜的生物物理研究表明,异丁二硫代氨基脲化合物(ITSC)可能改变Aβ聚集的过程,其中两种衍生物表现出显着的抑制聚集过程的能力,从而完全阻止了形成淀粉样原纤维。此外,在体外在原代神经元细胞培养的研究中,发现ITSC在低至1μM的浓度下抑制Aβ诱导的神经毒性和活性氧的产生。综上所述,可以将新型ITSC视为潜在的AD疗法,作为进一步发展的特权结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号