当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer Cell Macrophage Membrane Camouflaged Persistent Luminescent Nanoparticles for Imaging-Guided Photothermal Therapy of Colorectal Cancer
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-06-29 , DOI: 10.1021/acsanm.0c01433 Zhi-Hao Wang 1 , Jing-Min Liu 1 , Ning Zhao 1 , Chun-Yang Li 1 , Shi-Wen Lv 1 , Yaozhong Hu 1 , Huan Lv 1 , Di Wang 1 , Shuo Wang 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-06-29 , DOI: 10.1021/acsanm.0c01433 Zhi-Hao Wang 1 , Jing-Min Liu 1 , Ning Zhao 1 , Chun-Yang Li 1 , Shi-Wen Lv 1 , Yaozhong Hu 1 , Huan Lv 1 , Di Wang 1 , Shuo Wang 1
Affiliation
|
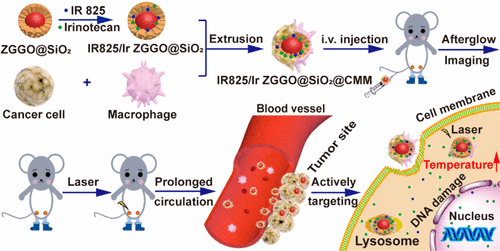
Colorectal cancer is one of the leading causes of cancer incidence and mortality worldwide. Early diagnosis and treatment of colorectal cancer is beneficial to improve the therapeutic efficacy and prolong the survival time of patients. In recent years, persistent luminescence nanoparticles (PLNPs) have attracted more and more attention in the field of biomedical imaging due to the long persistent luminescence, zero autofluorescence, and high signal-to-noise ratio. To real-time trace and diagnose colorectal cancer and produce a precise treatment effect, herein, Zn1.25Ga1.5Ge0.25O4:Cr3+,Yb3+,Er3+ (ZGGO) PLNPs were prepared and served as the trackable center and coated with mesoporous silica (ZGGO@SiO2). Afterward, photothermal fluorescent dye IR825 and chemotherapeutic drug irinotecan (Ir) were successively loaded into the mesoporous ZGGO@SiO2 and encapsulated with a cancer cell–macrophage hybrid membrane. With relatively uniform particle size, the hybrid membrane camouflaged nano delivery system (IR825/Ir ZGGO@SiO2@CMM) exhibited superior escape ability and homologous adhesion ability in in vitro cellular uptake experiment and in vivo persistent luminescence imaging experiment, favoring the long-term retaining of nanocarriers in vivo and targeted accumulating in tumor sites. In vivo antitumor experiment showed that the nanoplatform could produce precise combined colorectal cancer chemotherapy and imaging-guided photothermal therapy under the persistent luminescent guide of PLNPs. After 3 weeks of treatment, the relative tumor volume of CT26-tumor-bearing mice treated with IR825/Ir ZGGO@SiO2@CMM and laser irradiation was only one-fifth of that of uncoated IR825/Ir ZGGO@SiO2. With superior immune escape ability and tumor homologous adhesion ability, the cancer cell–macrophage hybrid membrane camouflaged nanomaterials may provide a universal nanoplatform that allows integrating various therapy modalities for precise cancer treatment of a biomimetic nano delivery system.
中文翻译:

癌细胞巨噬细胞膜伪装的持久发光纳米粒子的成像指导光热疗法治疗结直肠癌
大肠癌是全世界癌症发生率和死亡率的主要原因之一。大肠癌的早期诊断和治疗有利于提高治疗效果,延长患者的生存时间。近年来,由于长持续发光,零自发荧光和高信噪比,持续发光纳米颗粒(PLNP)在生物医学成像领域引起了越来越多的关注。为了实时追踪和诊断大肠癌并产生精确的治疗效果,此处使用Zn 1.25 Ga 1.5 Ge 0.25 O 4:Cr 3+,Yb 3+,Er 3+(ZGGO)制备PLNP并用作可追踪中心,并用中孔二氧化硅(ZGGO @ SiO 2)涂覆。然后,将光热荧光染料IR825和化学治疗药物伊立替康(Ir)依次装入中孔ZGGO @ SiO 2中,并用癌细胞-巨噬细胞杂化膜封装。具有相对均一的粒径的杂化膜伪装纳米递送系统(IR825 / Ir ZGGO @ SiO 2 @CMM)在体外细胞摄取实验和体内持续发光成像实验中表现出优异的逃逸能力和同源粘附能力,有利于长期体内纳米载体的长期保留并有针对性地聚集在肿瘤部位 体内抗肿瘤实验表明,在PLNPs持续发光的指导下,纳米平台可以产生精确的联合结直肠癌化学疗法和成像指导的光热疗法。治疗3周后,用IR825 / Ir ZGGO @ SiO 2 @CMM和激光照射治疗的携带CT26的荷瘤小鼠的相对肿瘤体积仅为未涂覆的IR825 / Ir ZGGO @ SiO 2的五分之一。具有卓越的免疫逃逸能力和肿瘤同源粘附能力的癌细胞-巨噬细胞杂化膜伪装纳米材料可以提供通用的纳米平台,该平台可以整合各种治疗方法,以精确地模拟仿生纳米递送系统进行癌症治疗。
更新日期:2020-06-29
中文翻译:

癌细胞巨噬细胞膜伪装的持久发光纳米粒子的成像指导光热疗法治疗结直肠癌
大肠癌是全世界癌症发生率和死亡率的主要原因之一。大肠癌的早期诊断和治疗有利于提高治疗效果,延长患者的生存时间。近年来,由于长持续发光,零自发荧光和高信噪比,持续发光纳米颗粒(PLNP)在生物医学成像领域引起了越来越多的关注。为了实时追踪和诊断大肠癌并产生精确的治疗效果,此处使用Zn 1.25 Ga 1.5 Ge 0.25 O 4:Cr 3+,Yb 3+,Er 3+(ZGGO)制备PLNP并用作可追踪中心,并用中孔二氧化硅(ZGGO @ SiO 2)涂覆。然后,将光热荧光染料IR825和化学治疗药物伊立替康(Ir)依次装入中孔ZGGO @ SiO 2中,并用癌细胞-巨噬细胞杂化膜封装。具有相对均一的粒径的杂化膜伪装纳米递送系统(IR825 / Ir ZGGO @ SiO 2 @CMM)在体外细胞摄取实验和体内持续发光成像实验中表现出优异的逃逸能力和同源粘附能力,有利于长期体内纳米载体的长期保留并有针对性地聚集在肿瘤部位 体内抗肿瘤实验表明,在PLNPs持续发光的指导下,纳米平台可以产生精确的联合结直肠癌化学疗法和成像指导的光热疗法。治疗3周后,用IR825 / Ir ZGGO @ SiO 2 @CMM和激光照射治疗的携带CT26的荷瘤小鼠的相对肿瘤体积仅为未涂覆的IR825 / Ir ZGGO @ SiO 2的五分之一。具有卓越的免疫逃逸能力和肿瘤同源粘附能力的癌细胞-巨噬细胞杂化膜伪装纳米材料可以提供通用的纳米平台,该平台可以整合各种治疗方法,以精确地模拟仿生纳米递送系统进行癌症治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号