当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impairing committed cholesterol biosynthesis in white matter astrocytes, but not grey matter astrocytes, enhances in vitro myelination
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-06-30 , DOI: 10.1111/jnc.15113 Inge L Werkman 1 , Janine Kövilein 1 , Jenny C de Jonge 1 , Wia Baron 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-06-30 , DOI: 10.1111/jnc.15113 Inge L Werkman 1 , Janine Kövilein 1 , Jenny C de Jonge 1 , Wia Baron 1
Affiliation
|
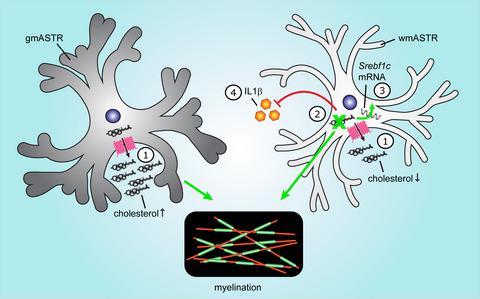
Remyelination is a regenerative process that is essential to recover saltatory conduction and to prevent neurodegeneration upon demyelination. The formation of new myelin involves the differentiation of oligodendrocyte progenitor cells (OPCs) toward oligodendrocytes and requires high amounts of cholesterol. Astrocytes (ASTRs) modulate remyelination by supplying lipids to oligodendrocytes. Remarkably, remyelination is more efficient in grey matter (GM) than in white matter (WM), which may relate to regional differences in ASTR subtype. Here, we show that a feeding layer of gmASTRs was more supportive to in vitro myelination than a feeding layer of wmASTRs. While conditioned medium from both gmASTRs and wmASTRs accelerated gmOPC differentiation, wmOPC differentiation is enhanced by secreted factors from gmASTRs, but not wmASTRs. In vitro analyses revealed that gmASTRs secreted more cholesterol than wmASTRs. Cholesterol efflux from both ASTR types was reduced upon exposure to pro‐inflammatory cytokines, which was mediated via cholesterol transporter ABCA1, but not ABCG1, and correlated with a minor reduction of myelin membrane formation by oligodendrocytes. Surprisingly, a wmASTR knockdown of Fdft1 encoding for squalene synthase (SQS), an enzyme essential for the first committed step in cholesterol biosynthesis, enhanced in vitro myelination. Reduced secretion of interleukin‐1β likely by enhanced isoprenylation, and increased unsaturated fatty acid synthesis, both pathways upstream of SQS, likely masked the effect of reduced levels of ASTR‐derived cholesterol. Hence, our findings indicate that gmASTRs export more cholesterol and are more supportive to myelination than wmASTRs, but specific inhibition of cholesterol biosynthesis in ASTRs is beneficial for wmASTR‐mediated modulation of myelination.
中文翻译:

损害白质星形胶质细胞而非灰质星形胶质细胞的胆固醇生物合成可增强体外髓鞘形成
髓鞘再生是一种再生过程,对于恢复跳跃传导和防止脱髓鞘时的神经变性至关重要。新髓磷脂的形成涉及少突胶质细胞祖细胞 (OPC) 向少突胶质细胞的分化,并且需要大量胆固醇。星形胶质细胞(ASTR)通过向少突胶质细胞提供脂质来调节髓鞘再生。值得注意的是,髓鞘再生在灰质 (GM) 中比在白质 (WM) 中更有效,这可能与 ASTR 亚型的区域差异有关。在这里,我们表明 gmASTR 的喂养层比 wmASTR 的喂养层更支持体外髓鞘形成。虽然来自 gmASTR 和 wmASTR 的条件培养基加速了 gmOPC 分化,但来自 gmASTR 而非 wmASTR 的分泌因子增强了 wmOPC 分化。体外分析表明,gmASTR 比 wmASTR 分泌更多的胆固醇。暴露于促炎细胞因子后,两种 ASTR 类型的胆固醇流出都会减少,这是通过胆固醇转运蛋白 ABCA1 介导的,但不是 ABCG1,并且与少突胶质细胞髓磷脂膜形成的轻微减少相关。令人惊讶的是,编码角鲨烯合酶(SQS)的Fdft1的 wmASTR 敲低增强了体外髓鞘形成,角鲨烯合酶是胆固醇生物合成第一个关键步骤所必需的酶。异戊二烯化增强可能导致白细胞介素-1β分泌减少,不饱和脂肪酸合成增加(SQS上游的两条途径)可能掩盖了 ASTR 衍生胆固醇水平降低的影响。因此,我们的研究结果表明,gmASTR 比 wmASTR 输出更多的胆固醇,并且更支持髓鞘形成,但 ASTR 中胆固醇生物合成的特异性抑制有利于 wmASTR 介导的髓鞘形成调节。
更新日期:2020-06-30
中文翻译:

损害白质星形胶质细胞而非灰质星形胶质细胞的胆固醇生物合成可增强体外髓鞘形成
髓鞘再生是一种再生过程,对于恢复跳跃传导和防止脱髓鞘时的神经变性至关重要。新髓磷脂的形成涉及少突胶质细胞祖细胞 (OPC) 向少突胶质细胞的分化,并且需要大量胆固醇。星形胶质细胞(ASTR)通过向少突胶质细胞提供脂质来调节髓鞘再生。值得注意的是,髓鞘再生在灰质 (GM) 中比在白质 (WM) 中更有效,这可能与 ASTR 亚型的区域差异有关。在这里,我们表明 gmASTR 的喂养层比 wmASTR 的喂养层更支持体外髓鞘形成。虽然来自 gmASTR 和 wmASTR 的条件培养基加速了 gmOPC 分化,但来自 gmASTR 而非 wmASTR 的分泌因子增强了 wmOPC 分化。体外分析表明,gmASTR 比 wmASTR 分泌更多的胆固醇。暴露于促炎细胞因子后,两种 ASTR 类型的胆固醇流出都会减少,这是通过胆固醇转运蛋白 ABCA1 介导的,但不是 ABCG1,并且与少突胶质细胞髓磷脂膜形成的轻微减少相关。令人惊讶的是,编码角鲨烯合酶(SQS)的Fdft1的 wmASTR 敲低增强了体外髓鞘形成,角鲨烯合酶是胆固醇生物合成第一个关键步骤所必需的酶。异戊二烯化增强可能导致白细胞介素-1β分泌减少,不饱和脂肪酸合成增加(SQS上游的两条途径)可能掩盖了 ASTR 衍生胆固醇水平降低的影响。因此,我们的研究结果表明,gmASTR 比 wmASTR 输出更多的胆固醇,并且更支持髓鞘形成,但 ASTR 中胆固醇生物合成的特异性抑制有利于 wmASTR 介导的髓鞘形成调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号