当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Co-expression with chaperones can affect protein 3D-structure as exemplified by loss-of-function variants of human prolidase
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-14 , DOI: 10.1002/1873-3468.13877 Elżbieta Wątor 1 , Maria Rutkiewicz 1 , Manfred S Weiss 1 , Piotr Wilk 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-14 , DOI: 10.1002/1873-3468.13877 Elżbieta Wątor 1 , Maria Rutkiewicz 1 , Manfred S Weiss 1 , Piotr Wilk 1
Affiliation
|
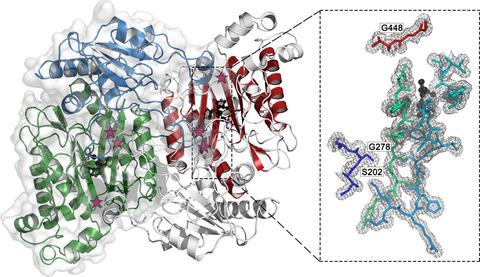
Prolidase catalyzes the cleavage of dipeptides containing proline on their C terminus. The reduction in prolidase activity is the cause of a rare disease named 'Prolidase Deficiency'. Local structural disorder was indicated as one of the causes for diminished prolidase activity. Previous studies showed that heat shock proteins can partially recover prolidase activity in vivo. To analyze this mechanism of enzymatic activity rescue, we compared the crystal structures of selected prolidase mutants expressed in the absence and in the presence of chaperones. Our results confirm that protein chaperones facilitate the formation of more ordered structures by their substrate protein. These results also suggest that the protein expression system needs to be considered as an important parameter in structural studies.
中文翻译:

与分子伴侣的共表达可以影响蛋白质 3D 结构,例如人类脯氨酸酶的功能丧失变体
脯氨酸酶催化在其 C 末端含有脯氨酸的二肽的裂解。脯氨酸酶活性的降低是一种名为“脯氨酸酶缺乏症”的罕见疾病的原因。局部结构紊乱被认为是脯氨酸酶活性降低的原因之一。先前的研究表明,热休克蛋白可以在体内部分恢复脯氨酸酶的活性。为了分析这种酶活性拯救的机制,我们比较了在没有和存在分子伴侣的情况下表达的所选脯氨酸酶突变体的晶体结构。我们的结果证实,蛋白质伴侣促进其底物蛋白质形成更有序的结构。这些结果还表明,蛋白质表达系统需要被视为结构研究中的一个重要参数。
更新日期:2020-07-14
中文翻译:

与分子伴侣的共表达可以影响蛋白质 3D 结构,例如人类脯氨酸酶的功能丧失变体
脯氨酸酶催化在其 C 末端含有脯氨酸的二肽的裂解。脯氨酸酶活性的降低是一种名为“脯氨酸酶缺乏症”的罕见疾病的原因。局部结构紊乱被认为是脯氨酸酶活性降低的原因之一。先前的研究表明,热休克蛋白可以在体内部分恢复脯氨酸酶的活性。为了分析这种酶活性拯救的机制,我们比较了在没有和存在分子伴侣的情况下表达的所选脯氨酸酶突变体的晶体结构。我们的结果证实,蛋白质伴侣促进其底物蛋白质形成更有序的结构。这些结果还表明,蛋白质表达系统需要被视为结构研究中的一个重要参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号