当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New synthesis of 6′′-[18 F]fluoromaltotriose for positron emission tomography (PET) imaging of bacterial infection
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-07-21 , DOI: 10.1002/jlcr.3868 Moustafa T Gabr 1, 2 , Tom Haywood 1, 2 , Gayatri Gowrishankar 1, 2 , Ananth Srinivasan 1, 2 , Sanjiv S Gambhir 1, 2, 3, 4
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-07-21 , DOI: 10.1002/jlcr.3868 Moustafa T Gabr 1, 2 , Tom Haywood 1, 2 , Gayatri Gowrishankar 1, 2 , Ananth Srinivasan 1, 2 , Sanjiv S Gambhir 1, 2, 3, 4
Affiliation
|
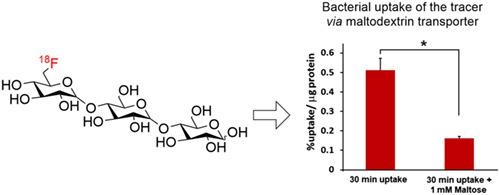
6''-[18 F]fluoromaltotriose is a positron emission tomography (PET) tracer that can differentiate between bacterial infection and inflammation in vivo. Bacteria-specific uptake of 6''-[18 F]fluoromaltotriose is attributed to the targeting of maltodextrin transporter in bacteria that is absent in mammalian cells. Herein, we report a new synthesis of 6''-[18 F]fluoromaltotriose as a key step for its clinical translation. In comparison to the previously reported synthesis, the new synthesis features unambiguous assignment of the fluorine-18 position on the maltotriose unit. The new method utilizes direct fluorination of 2'',3'',4''-tri-O-acetyl-6''-O-trifyl-α-D-glucopyranosyl-(1-4)-O-2',3',6'-tri-O-acetyl-α-D-glucopyranosyl-(1-4)-1,2,3,6-tetra-O-acetyl-D-glucopyranose followed by basic hydrolysis. Radiolabeling of the new maltotriose triflate precursor proceeds using a single HPLC purification step, which results in shorter reaction time in comparison to the previously reported synthesis. Successful synthesis of 6''-[18 F]fluoromaltotriose has been achieved in 3.5 ± 0.3% radiochemical yield (decay corrected, n=7) and radiochemical purity above 95%. The efficient radiosynthesis of 6''-[18 F]fluoromaltotriose would be critical in advancing this PET tracer into clinical trials for imaging bacterial infections.
中文翻译:

用于细菌感染正电子发射断层扫描(PET)成像的 6′′-[18 F]氟麦芽三糖的新合成
6''-[18 F]氟麦芽三糖是一种正电子发射断层扫描 (PET) 示踪剂,可以区分体内细菌感染和炎症。 6''-[18 F]氟麦芽三糖的细菌特异性摄取归因于细菌中麦芽糖糊精转运蛋白的靶向,而哺乳动物细胞中不存在这种转运蛋白。在此,我们报告了 6''-[18 F]氟麦芽三糖的新合成,作为其临床转化的关键步骤。与之前报道的合成相比,新的合成具有麦芽三糖单元上氟 18 位置的明确分配。新方法利用2'',3'',4''-三-O-乙酰基-6''-O-三氟甲酰-α-D-吡喃葡萄糖基-(1-4)-O-2'的直接氟化, 3',6'-三-O-乙酰基-α-D-吡喃葡萄糖基-(1-4)-1,2,3,6-四-O-乙酰基-D-吡喃葡萄糖,然后进行碱性水解。新的麦芽三糖三氟甲磺酸酯前体的放射性标记使用单个 HPLC 纯化步骤进行,与之前报道的合成相比,这导致反应时间更短。 6''-[18 F]氟麦芽三糖的成功合成已实现,放射化学产率为 3.5 ± 0.3%(衰减校正,n=7),放射化学纯度高于 95%。 6''-[18 F]氟麦芽三糖的有效放射合成对于将该 PET 示踪剂推进细菌感染成像临床试验至关重要。
更新日期:2020-07-21
中文翻译:

用于细菌感染正电子发射断层扫描(PET)成像的 6′′-[18 F]氟麦芽三糖的新合成
6''-[18 F]氟麦芽三糖是一种正电子发射断层扫描 (PET) 示踪剂,可以区分体内细菌感染和炎症。 6''-[18 F]氟麦芽三糖的细菌特异性摄取归因于细菌中麦芽糖糊精转运蛋白的靶向,而哺乳动物细胞中不存在这种转运蛋白。在此,我们报告了 6''-[18 F]氟麦芽三糖的新合成,作为其临床转化的关键步骤。与之前报道的合成相比,新的合成具有麦芽三糖单元上氟 18 位置的明确分配。新方法利用2'',3'',4''-三-O-乙酰基-6''-O-三氟甲酰-α-D-吡喃葡萄糖基-(1-4)-O-2'的直接氟化, 3',6'-三-O-乙酰基-α-D-吡喃葡萄糖基-(1-4)-1,2,3,6-四-O-乙酰基-D-吡喃葡萄糖,然后进行碱性水解。新的麦芽三糖三氟甲磺酸酯前体的放射性标记使用单个 HPLC 纯化步骤进行,与之前报道的合成相比,这导致反应时间更短。 6''-[18 F]氟麦芽三糖的成功合成已实现,放射化学产率为 3.5 ± 0.3%(衰减校正,n=7),放射化学纯度高于 95%。 6''-[18 F]氟麦芽三糖的有效放射合成对于将该 PET 示踪剂推进细菌感染成像临床试验至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号