当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral anionic layers in tartramide spiroborate salts and variable solvation for [NR4][B(TarNH2)2] (R = Et, Pr or Bu).
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-06-30 , DOI: 10.1107/s2053229620008384 Aristyo Soecipto 1 , Lawrence W Y Wong 1 , Herman H Y Sung 1 , Ian D Williams 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-06-30 , DOI: 10.1107/s2053229620008384 Aristyo Soecipto 1 , Lawrence W Y Wong 1 , Herman H Y Sung 1 , Ian D Williams 1
Affiliation
|
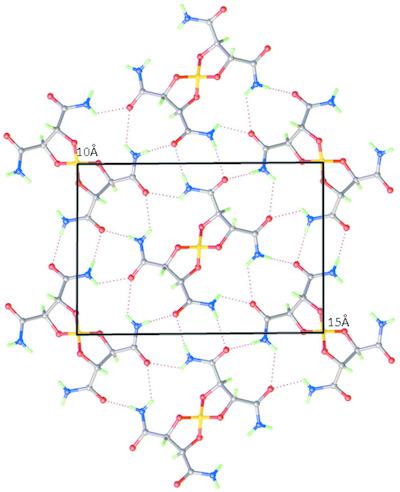
The spiroborate anion, namely, 2,3,7,8‐tetracarboxamido‐1,4,6,9‐tetraoxa‐5λ4‐boraspiro[4.4]nonane, [B(TarNH2)2]−, derived from the diol l‐tartramide TarNH2, [CH(O)(CONH2)]2, shows a novel self‐assembly into two‐dimensional (2D) layer structures in its salts with alkylammonium cations, [NR4]+ (R = Et, Pr and Bu), and sparteinium, [HSpa]+, in which the cations and anions are segregated. The structures of four such salts are reported, namely, the tetrapropylazanium salt, C12H28N+·C8H12BN4O8−, the tetraethylazanium salt hydrate, C8H20N+·C8H12BN4O8−·6.375H2O, the tetrabutylazanium salt as the ethanol monosolvate hemihydrate, C16H36N+·C8H12BN4O8−·C2H5OH·0.5H2O, and the sparteinium (7‐aza‐15‐azoniatetracyclo[7.7.1.02,7.010,15]heptadecane) salt as the ethanol monosolvate, C15H27N2+·C8H12BN4O8−·C2H5OH. The 2D anion layers have preserved intermolecular hydrogen bonding between the amide groups and a typical metric repeat of around 10 × 15 Å. The constraint of matching the interfacial area organizes the cations into quite different solvated arrangements, i.e. the [NEt4] salt is highly hydrated with around 6.5H2O per cation, the [NPr4] salt apparently has a good metric match to the anion layer and is unsolvated, whilst the [NBu4] salt is intermediate and has EtOH and H2O in its cation layer, which is similar to the arrangement for the chiral [HSpa]+ cation. This family of salts shows highly organized chiral space and offers potential for the resolution of both chiral cations and neutral chiral solvent molecules.
中文翻译:

酒石酰胺螺硼酸盐中的手性阴离子层和对[NR4] [B(TarNH2)2](R = Et,Pr或Bu)的可变溶剂化。
的spiroborate阴离子,即2,3,7,8- tetracarboxamido-1,4,6,9四氧5λ 4 -boraspiro [4.4]壬烷,[B(TarNH 2)2 ] - ,从二醇衍生升‐tartramide TarNH 2,[CH(O)(CONH 2)] 2,在其与烷基铵阳离子[N R 4 ] +(R = Et, Pr和Bu),以及天竺葵[HSpa] +,其中的阳离子和阴离子被隔离。报道了四种盐的结构,即四丙基氮杂盐,C 12 H 28ñ + ·C 8 H ^ 12 BN 4 ø 8 -时,tetraethylazanium盐水合物,C 8 ħ 20 ñ + ·C 8 H ^ 12 BN 4 ö 8 - ·6.375H 2 O,所述tetrabutylazanium盐作为乙醇单溶剂半水合物,C 16 H 36 N + ·C 8 H 12 BN 4 O 8 − ·C 2 H 5 OH·0.5H 2O和作为乙醇单溶剂化物的盐(7-氮杂-15-氮杂四环[7.7.1.0 2,7 .0 10,15 ]十七烷),C 15 H 27 N 2 + ·C 8 H 12 BN 4 O 8 - ·C 2 H 5 OH。2D阴离子层保留了酰胺基团之间的分子间氢键,典型的重复量约为10×15Å。匹配界面区域的约束将阳离子组织成完全不同的溶剂化排列,即[NEt 4 ]盐在大约6.5H 2的高度水合对于每个阳离子,[NPr 4 ]盐显然与阴离子层具有良好的度量匹配,并且是非溶剂化的;而[NBu 4 ]盐处于中间状态,并且在其阳离子层中具有EtOH和H 2 O,这与阴离子层相似。手性[HSpa] +阳离子的排列。该盐家族显示出高度有序的手性空间,并为拆分手性阳离子和中性手性溶剂分子提供了潜力。
更新日期:2020-06-30
中文翻译:

酒石酰胺螺硼酸盐中的手性阴离子层和对[NR4] [B(TarNH2)2](R = Et,Pr或Bu)的可变溶剂化。
的spiroborate阴离子,即2,3,7,8- tetracarboxamido-1,4,6,9四氧5λ 4 -boraspiro [4.4]壬烷,[B(TarNH 2)2 ] - ,从二醇衍生升‐tartramide TarNH 2,[CH(O)(CONH 2)] 2,在其与烷基铵阳离子[N R 4 ] +(R = Et, Pr和Bu),以及天竺葵[HSpa] +,其中的阳离子和阴离子被隔离。报道了四种盐的结构,即四丙基氮杂盐,C 12 H 28ñ + ·C 8 H ^ 12 BN 4 ø 8 -时,tetraethylazanium盐水合物,C 8 ħ 20 ñ + ·C 8 H ^ 12 BN 4 ö 8 - ·6.375H 2 O,所述tetrabutylazanium盐作为乙醇单溶剂半水合物,C 16 H 36 N + ·C 8 H 12 BN 4 O 8 − ·C 2 H 5 OH·0.5H 2O和作为乙醇单溶剂化物的盐(7-氮杂-15-氮杂四环[7.7.1.0 2,7 .0 10,15 ]十七烷),C 15 H 27 N 2 + ·C 8 H 12 BN 4 O 8 - ·C 2 H 5 OH。2D阴离子层保留了酰胺基团之间的分子间氢键,典型的重复量约为10×15Å。匹配界面区域的约束将阳离子组织成完全不同的溶剂化排列,即[NEt 4 ]盐在大约6.5H 2的高度水合对于每个阳离子,[NPr 4 ]盐显然与阴离子层具有良好的度量匹配,并且是非溶剂化的;而[NBu 4 ]盐处于中间状态,并且在其阳离子层中具有EtOH和H 2 O,这与阴离子层相似。手性[HSpa] +阳离子的排列。该盐家族显示出高度有序的手性空间,并为拆分手性阳离子和中性手性溶剂分子提供了潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号