当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-uniformly sampled rare-spin 4D solid state NMR experiments: assignment and characterization of IKe phage capsid
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-08-19 , DOI: 10.1002/mrc.5072 Gal Porat 1, 2 , Orr Simon Lusky 1 , Nir Dayan 1, 3 , Amir Goldbourt 1
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-08-19 , DOI: 10.1002/mrc.5072 Gal Porat 1, 2 , Orr Simon Lusky 1 , Nir Dayan 1, 3 , Amir Goldbourt 1
Affiliation
|
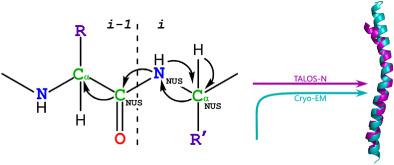
An important step in the process of protein research by NMR is the assignment of chemical shifts. In the coat protein of IKe bacteriophage there are 53 residues making up a long helix resulting in relatively high spectral ambiguity. Assignment thus requires the collection of a set of three-dimensional (3D) experiments and the preparation of sparsely labeled samples. Increasing the dimensionality can facilitate fast and reliable assignment of IKe and of larger proteins. Recent progress in non-uniform sampling techniques made the application of multi-dimensional NMR solid-state experiments beyond 3D more practical. 4D 1 H-detected experiments have been demonstrated in high-fields and at spinning speeds of 60 kHz and higher, but are not practical at spinning speeds of 10-20 kHz for fully-protonated proteins. Here we demonstrate the applicability of a non-uniformly sampled 4D 13 C/15 N-only correlation experiment performed at a moderate field of 14.1T, which can incorporate sufficiently long acquisition periods in all dimensions. We demonstrate how a single CANCOCX experiment, supported by several 2D carbon-based correlation experiments, is utilized for the assignment of heteronuclei in the coat protein of the IKe bacteriophage. One sparsely-labeled sample was used to validate sidechain assignment of several hydrophobic-residue sidechains. A comparison to solution NMR studies of isolated IKe coat proteins embedded in micelles points to key residues involved in structural rearrangement of the capsid upon assembly of the virus. The benefits of 4D to a quicker assignment are discussed, and the method may prove useful for studying proteins at relatively low fields.
中文翻译:

非均匀采样的稀有自旋 4D 固态 NMR 实验:IKe 噬菌体衣壳的分配和表征
NMR 蛋白质研究过程中的一个重要步骤是化学位移的分配。在 IKe 噬菌体的外壳蛋白中有 53 个残基组成一个长螺旋,导致相对较高的光谱模糊度。因此,分配需要收集一组三维 (3D) 实验并准备稀疏标记的样本。增加维度可以促进 IKE 和更大蛋白质的快速和可靠分配。非均匀采样技术的最新进展使 3D 以外的多维 NMR 固态实验的应用更加实用。4D 1 H 检测实验已经在高场和 60 kHz 及更高的旋转速度下进行了证明,但对于完全质子化的蛋白质,在 10-20 kHz 的旋转速度下并不实用。在这里,我们展示了在 14.1T 中等场强下进行的非均匀采样 4D 13 C/15 N-only 相关实验的适用性,该实验可以在所有维度上包含足够长的采集周期。我们展示了如何通过几个基于二维碳的相关实验支持的单个 CANCOCX 实验用于分配 IKe 噬菌体外壳蛋白中的异核。一个稀疏标记的样品用于验证几个疏水残基侧链的侧链分配。与嵌入胶束中的分离的 IKE 外壳蛋白的溶液 NMR 研究的比较指出了在病毒组装时参与衣壳结构重排的关键残基。讨论了 4D 对更快分配的好处,
更新日期:2020-08-19
中文翻译:

非均匀采样的稀有自旋 4D 固态 NMR 实验:IKe 噬菌体衣壳的分配和表征
NMR 蛋白质研究过程中的一个重要步骤是化学位移的分配。在 IKe 噬菌体的外壳蛋白中有 53 个残基组成一个长螺旋,导致相对较高的光谱模糊度。因此,分配需要收集一组三维 (3D) 实验并准备稀疏标记的样本。增加维度可以促进 IKE 和更大蛋白质的快速和可靠分配。非均匀采样技术的最新进展使 3D 以外的多维 NMR 固态实验的应用更加实用。4D 1 H 检测实验已经在高场和 60 kHz 及更高的旋转速度下进行了证明,但对于完全质子化的蛋白质,在 10-20 kHz 的旋转速度下并不实用。在这里,我们展示了在 14.1T 中等场强下进行的非均匀采样 4D 13 C/15 N-only 相关实验的适用性,该实验可以在所有维度上包含足够长的采集周期。我们展示了如何通过几个基于二维碳的相关实验支持的单个 CANCOCX 实验用于分配 IKe 噬菌体外壳蛋白中的异核。一个稀疏标记的样品用于验证几个疏水残基侧链的侧链分配。与嵌入胶束中的分离的 IKE 外壳蛋白的溶液 NMR 研究的比较指出了在病毒组装时参与衣壳结构重排的关键残基。讨论了 4D 对更快分配的好处,











































 京公网安备 11010802027423号
京公网安备 11010802027423号