当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Remote Substituents on the Torquoselectivity of 3-Silyl Cyclobutene-Derivatives Ring-Opening Reactions.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-21 , DOI: 10.1002/cphc.202000451 Olatz Larrañaga 1 , Abel de Cózar 1, 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-07-21 , DOI: 10.1002/cphc.202000451 Olatz Larrañaga 1 , Abel de Cózar 1, 2
Affiliation
|
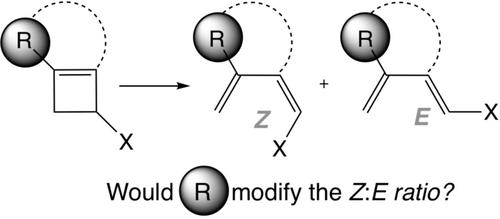
We have quantum chemically studied the structure and nature of 1‐substituted 3‐trimethylsilyl cyclobutenes and 2,2‐disubstituted‐7‐trimethylsilyl bycyclo[4.2.0]octa‐1(6),3‐dienes comparing their reactivity to archetypal 3‐substituted cyclobutene systems using density functional theory at M06‐2X(PCM)/TZ2P level. We wish to understand how the ring opening reaction is affected by a substituent not directly connected to the σ Carbon‐Carbon breaking bond. To this end, we have analyzed the reaction profiles considering second order perturbation energies and natural steric analysis within NBO framework.
中文翻译:

远程取代基对3-甲硅烷基环丁烯-衍生物开环反应的四聚体选择性的影响。
我们已经用量子化学方法通过环[4.2.0] octa-1(6),3-二烯研究了1-取代的3-三甲基甲硅烷基环丁烯和2,2-二取代的-7-三甲基甲硅烷基的结构和性质,比较了它们与原型3-使用密度泛函理论在M06-2X(PCM)/ TZ2P级别上取代环丁烯系统。我们希望了解开环反应如何受到未直接连接到σ碳-碳断裂键的取代基的影响。为此,我们在NBO框架内考虑了二级扰动能量和自然空间分析,分析了反应曲线。
更新日期:2020-07-21
中文翻译:

远程取代基对3-甲硅烷基环丁烯-衍生物开环反应的四聚体选择性的影响。
我们已经用量子化学方法通过环[4.2.0] octa-1(6),3-二烯研究了1-取代的3-三甲基甲硅烷基环丁烯和2,2-二取代的-7-三甲基甲硅烷基的结构和性质,比较了它们与原型3-使用密度泛函理论在M06-2X(PCM)/ TZ2P级别上取代环丁烯系统。我们希望了解开环反应如何受到未直接连接到σ碳-碳断裂键的取代基的影响。为此,我们在NBO框架内考虑了二级扰动能量和自然空间分析,分析了反应曲线。











































 京公网安备 11010802027423号
京公网安备 11010802027423号