当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selenide-chitosan as High-performance Nanophotocatalyst for Accelerated Degradation of Pollutants.
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-06-29 , DOI: 10.1002/asia.202000597 Nisar Ali 1 , Shehzad Ahmad 2 , Adnan Khan 2 , Saraf Khan 2 , Muhammad Bilal 3 , Salah Ud Din 2 , Nauman Ali 2 , Hafiz M N Iqbal 4 , Hammad Khan 5
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-06-29 , DOI: 10.1002/asia.202000597 Nisar Ali 1 , Shehzad Ahmad 2 , Adnan Khan 2 , Saraf Khan 2 , Muhammad Bilal 3 , Salah Ud Din 2 , Nauman Ali 2 , Hafiz M N Iqbal 4 , Hammad Khan 5
Affiliation
|
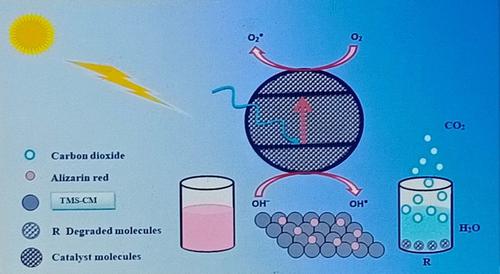
Water pollution is one of the major global challenges today. Water bodies are contaminated by the heavy release of waste effluents of textile industries, which includes intensively colored dye pollutants. Herein, a ternary nanocomposite of bismuth copper selenide with small particle size and ternary metal selenide (TMS)‐chitosan microspheres (TMS‐CM) of the spherical porous surface were successfully synthesized. SEM, XRD, EDX, FTIR, and UV/Vis spectrophotometry analysis revealed the structural and morphological characteristics of the newly synthesized nanocomposites. SEM imaging showed the average diameter of TMS nanoparticle to be 33 nm. The crystal size was calculated as 6.33 nm and crystalline structure as orthorhombic using XRD findings. EDX confirmed the presence of Bi, Cu, and Se in the ternary nanocomposite. The bandgap of 1.8 eV was calculated from Tauc's plot for the TMS nanocomposite. SEM confirmed the successful synthesis of spherical TMS‐CM microspheres of porous surface morphology with an average size of 885.6 μm. The presence of chitosan microspheres in the synthesis of TMS nanocomposite was identified by FTIR spectral analysis. Furthermore, highly efficient photocatalytic degradation (up to 95.4%) of ARS was achieved within 180 min at pH 4.0 using 0.5 g of TMS‐CM in sunlight. The first‐order kinetic model fitted well to the photocatalytic decontamination of ARS using TMS‐CM with a rate constant of 6.1x10−2 min−1. The TMS‐CM gave attractive results and high efficiency in photocatalytic degradation of ARS dye after reusing and regeneration of up to seven successive cycles. The newly synthesized nanophotocatalyst could be efficiently used for the decontamination of dye polluted water from textile industries.
中文翻译:

硒化壳聚糖作为促进污染物降解的高性能纳米光催化剂。
水污染是当今全球面临的主要挑战之一。水体被纺织工业产生的大量废水排放所污染,其中包括强烈着色的染料污染物。在此,成功地合成了小粒径硒化铋铜的三元纳米复合材料和球形多孔表面的三元金属硒化物(TMS)-壳聚糖微球(TMS-CM)。SEM,XRD,EDX,FTIR和UV / Vis分光光度法分析揭示了新合成的纳米复合材料的结构和形态特征。SEM成像显示TMS纳米颗粒的平均直径为33nm。利用XRD发现,晶体尺寸计算为6.33nm,晶体结构为正交晶体。EDX证实三元纳米复合材料中存在Bi,Cu和Se。带隙为1。从Tauc的TMS纳米复合材料图计算得出8 eV。SEM证实了球形表面形态的球形TMS-CM微球的成功合成,其平均粒径为885.6μm。通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在阳光下使用0.5 g TMS-CM在pH 4.0下180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10 通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在阳光下使用0.5 g TMS-CM在pH 4.0下180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10 通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在pH 4.0下使用0.5 g TMS-CM在阳光下,在180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10−2 min -1。在重复使用和再生多达七个连续循环后,TMS-CM可以在ARS染料的光催化降解方面提供引人注目的结果和高效率。新合成的纳米光催化剂可以有效地用于纺织工业染料污染水的去污。
更新日期:2020-09-01
中文翻译:

硒化壳聚糖作为促进污染物降解的高性能纳米光催化剂。
水污染是当今全球面临的主要挑战之一。水体被纺织工业产生的大量废水排放所污染,其中包括强烈着色的染料污染物。在此,成功地合成了小粒径硒化铋铜的三元纳米复合材料和球形多孔表面的三元金属硒化物(TMS)-壳聚糖微球(TMS-CM)。SEM,XRD,EDX,FTIR和UV / Vis分光光度法分析揭示了新合成的纳米复合材料的结构和形态特征。SEM成像显示TMS纳米颗粒的平均直径为33nm。利用XRD发现,晶体尺寸计算为6.33nm,晶体结构为正交晶体。EDX证实三元纳米复合材料中存在Bi,Cu和Se。带隙为1。从Tauc的TMS纳米复合材料图计算得出8 eV。SEM证实了球形表面形态的球形TMS-CM微球的成功合成,其平均粒径为885.6μm。通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在阳光下使用0.5 g TMS-CM在pH 4.0下180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10 通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在阳光下使用0.5 g TMS-CM在pH 4.0下180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10 通过FTIR光谱分析鉴定了壳聚糖微球在TMS纳米复合材料的合成中的存在。此外,在pH 4.0下使用0.5 g TMS-CM在阳光下,在180分钟内即可实现ARS的高效光催化降解(高达95.4%)。一阶动力学模型非常适合使用TMS-CM进行ARS的光催化净化,速率常数为6.1x10−2 min -1。在重复使用和再生多达七个连续循环后,TMS-CM可以在ARS染料的光催化降解方面提供引人注目的结果和高效率。新合成的纳米光催化剂可以有效地用于纺织工业染料污染水的去污。



























 京公网安备 11010802027423号
京公网安备 11010802027423号