当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Coordination Chemistry of the N-Donor Substituted Phosphazanes Alex J. Plajer,a* Andrew D. Bond a and Dominic S. Wright a.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/chem.202002693 Alex J Plajer 1 , Andrew D Bond 1 , Dominic S Wright 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-06-30 , DOI: 10.1002/chem.202002693 Alex J Plajer 1 , Andrew D Bond 1 , Dominic S Wright 1
Affiliation
|
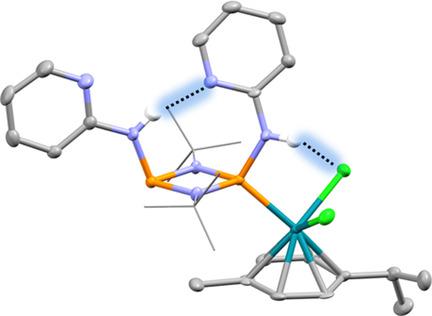
Phosph(III)azanes, featuring the heterocyclobutane P2N2 ring, have now been established as building blocks in main‐group coordination and supramolecular compounds. Previous studies have largely involved their use as neutral P‐donor ligands or as anionic N‐donor ligands, derived from deprotonation of amido‐phosphazanes [RNHP(μ‐NR)]2. The use of neutral amido‐phosphazanes themselves as chelating, H‐bond donors in anion receptors has also been an area of recent interest because of the ease by which the proton acidity and anion binding constants can be modulated, by the incorporation of electron‐withdrawing exo‐ and endo‐cyclic groups (R) and by the coordination of transition metals to the ring P atoms. We observed recently that the effect of P,N‐chelation of metal atoms to the P atoms of cis‐[(2‐py)NHP(μ‐NtBu)]2 (2‐py=2‐pyridyl) not only pre‐organises the N−H functionality for optimum H‐bonding to anions but also results in a large increase in anion binding constants, well above those for traditional organic receptors like squaramides and ureas. Here, we report a broader investigation of ligand chemistry of [(2‐py)NHP(μ‐tNBu)]2 (and of the new quinolyl derivative [(8‐Qu)NHP(μ‐NtBu)]2 (8‐Qu=8‐quinolyl). The additional N‐donor functionality of the heterocyclic substituents and its position has a marked effect on the anion and metal coordination chemistry of both species, leading to novel structural behaviour and reactivity compared to unfunctionalized counterparts.
中文翻译:

N-供体取代的Phosphazanes的配位化学Alex J. Plajer,a * Andrew D.Bond a和Dominic S.Wright a。
具有杂环丁烷P 2 N 2环的磷酸(III)氮烷现已被确立为主基团配位和超分子化合物的组成部分。先前的研究主要涉及将其用作中性P-供体配体或阴离子N-供体配体,它们是由酰胺基磷氮烷[RNHP(μ-NR)] 2的去质子化衍生的。使用中性酰胺基磷氮烷本身作为阴离子受体的H键供体的螯合剂,也很受关注,因为通过引入吸电子,可以很容易地调节质子酸度和阴离子结合常数。外和内环基团(R)和过渡金属与环P原子的配位。最近,我们观察到,P,金属原子的P原子的N-二螯合的作用顺- [(2-吡啶)NHP(μ-N吨丁基)] 2(2-PY = 2-吡啶基)不仅预重新组织了N-H功能,以实现与阴离子的最佳H键合,但也导致阴离子结合常数大大增加,远高于传统有机受体(如方胺和尿素)的结合常数。这里,我们报告的[(2-吡啶)NHP(μ-配体化学的更广泛的调查吨NBU)] 2(和新的喹啉衍生物的[(8-曲)NHP(μ-N吨丁基)] 2(8-Qu = 8-喹啉基)。杂环取代基的额外N供体官能团及其位置对两个物种的阴离子和金属配位化学都有显着影响,与未官能化的对应物相比,具有新颖的结构行为和反应活性。
更新日期:2020-06-30
中文翻译:

N-供体取代的Phosphazanes的配位化学Alex J. Plajer,a * Andrew D.Bond a和Dominic S.Wright a。
具有杂环丁烷P 2 N 2环的磷酸(III)氮烷现已被确立为主基团配位和超分子化合物的组成部分。先前的研究主要涉及将其用作中性P-供体配体或阴离子N-供体配体,它们是由酰胺基磷氮烷[RNHP(μ-NR)] 2的去质子化衍生的。使用中性酰胺基磷氮烷本身作为阴离子受体的H键供体的螯合剂,也很受关注,因为通过引入吸电子,可以很容易地调节质子酸度和阴离子结合常数。外和内环基团(R)和过渡金属与环P原子的配位。最近,我们观察到,P,金属原子的P原子的N-二螯合的作用顺- [(2-吡啶)NHP(μ-N吨丁基)] 2(2-PY = 2-吡啶基)不仅预重新组织了N-H功能,以实现与阴离子的最佳H键合,但也导致阴离子结合常数大大增加,远高于传统有机受体(如方胺和尿素)的结合常数。这里,我们报告的[(2-吡啶)NHP(μ-配体化学的更广泛的调查吨NBU)] 2(和新的喹啉衍生物的[(8-曲)NHP(μ-N吨丁基)] 2(8-Qu = 8-喹啉基)。杂环取代基的额外N供体官能团及其位置对两个物种的阴离子和金属配位化学都有显着影响,与未官能化的对应物相比,具有新颖的结构行为和反应活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号