Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.tetlet.2020.152173 Virginia V. Triantakonstanti , Alexandros Toskas , Nicolaos S. Iordanidis , Thanos Andreou , Theoharis V. Koftis , John K. Gallos
|
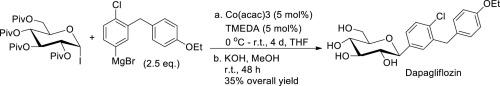
A method for the selective synthesis of β-C-glucosides using α-D-tetra-O-pivaloylglucosyl iodide as a glucosyl donor is reported. Its diastereoselectivity differs from that of the respective acetyl-protected glucosyl bromide, as it reported in the literature under similar reaction conditions. The concentration of the catalyst, the solvent and the type of additive used are crucial factors that determine the reaction selectivity. This method has been applied in a short synthesis of dapagliflozin. The stability of α-D-tetra-O-pivaloylglucosyl iodide in CDCl3 and THF at reflux was also studied. All side products in the coupling and decomposition reactions were isolated and characterized, and possible pathways for their formation are proposed.
中文翻译:

新戊酰保护的葡糖基碘化物作为葡糖基供体,用于制备β - C-葡糖苷
报道了一种使用α - D-四-O-新戊酰葡糖基碘化物作为葡糖基供体来选择性合成β - C-葡糖苷的方法。如文献中报道的,在相似的反应条件下,它的非对映选择性不同于相应的乙酰基保护的葡糖基溴。催化剂的浓度,溶剂和所用添加剂的类型是决定反应选择性的关键因素。该方法已用于dapagliflozin的短合成中。α - D-四-O-新戊酰葡萄糖基碘在CDCl 3中的稳定性还研究了回流下的THF。分离和表征了偶联和分解反应中的所有副产物,并提出了其形成的可能途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号