当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of C(9) hydroxy analogues of hederacines A and B
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.tetlet.2020.152170 Kang Yee Seah , Jeremy Robertson
中文翻译:

对映体合成C(9)的二十碳六烯酸A和B的羟基类似物
更新日期:2020-08-27
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.tetlet.2020.152170 Kang Yee Seah , Jeremy Robertson
|
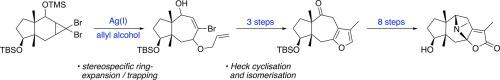
A strategy for assembling the 5-7-5 fused ring system of the hederacine alkaloids is reported, based on a sequence of electrocyclic ring expansion, with trapping in situ, then Heck cyclisation. Subsequent furan oxidative N-cyclisation generates the azabicyclo[3.2.1]octane core, resulting in a synthesis of C(9) hydroxy analogues of hederacines A and B in 19 and 20 steps, respectively, from 2-methylcyclopentane-1,3-dione.
中文翻译:

对映体合成C(9)的二十碳六烯酸A和B的羟基类似物
据报道,基于一系列电环扩环,原位捕获,然后进行Heck环化的方法,组装了二十二烷生物碱的5-7-5稠环系统的策略。随后的呋喃氧化N-环化生成氮杂双环[3.2.1]辛烷核,分别由19个和20个步骤由2-甲基环戊烷-1,3-合成19个和20个步骤的二十碳三烯A和B的C(9)羟基类似物。 dione。











































 京公网安备 11010802027423号
京公网安备 11010802027423号