Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.jmb.2020.06.012 Ahmed Rohaim 1 , LiDong Gong 2 , Jing Li 3 , Huan Rui 3 , Lydia Blachowicz 3 , Benoît Roux 3
|
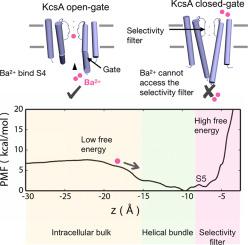
Barium (Ba2+) is a classic permeant blocker of potassium (K+) channels. The “external lock-in effect” in barium block experiments, whereby the binding of external K+ impedes the forward translocation of the blocker, provides a powerful avenue to investigate the selectivity of the binding sites along the pore of potassium channels. Barium block experiments show that the external lock-in site is highly selective for K+ over Na+. Wild-type KcsA was crystallized in low K+ conditions, and the crystals were soaked in solutions containing various concentrations of barium. Structural analysis reveals open and closed gate conformations of the KcsA channel. Anomalous diffraction experiments show that Ba2+ primarily binds to the innermost site S4 of the selectivity filter of the open-gate conformation and also the site S2, but no binding is detected with the closed-gate conformation. Alchemical free-energy perturbation calculations indicate that the presence of a Ba2+ ion in the selectivity filter boosts the specificity of K+ binding relative to Na+ in the external sites S0–S2.
中文翻译:

钡阻钾通道的开闭结构。
钡 (Ba 2+ ) 是钾 (K + ) 通道的经典渗透阻滞剂。钡阻滞实验中的“外部锁定效应”,即外部 K +的结合阻碍了阻滞剂的正向易位,为研究沿钾通道孔的结合位点的选择性提供了有力的途径。钡块实验表明,与Na + 相比,外部锁定位点对 K +具有高度选择性。野生型 KcsA 在低 K + 中结晶条件,并将晶体浸泡在含有不同浓度钡的溶液中。结构分析揭示了 KcsA 通道的开放和封闭门构象。异常衍射实验表明,Ba 2+主要与开门构象的选择性过滤器的最内位点S4以及位点S2结合,但未检测到与闭门构象的结合。炼金术自由能扰动计算表明,选择性过滤器中Ba 2+离子的存在提高了 K +相对于外部位点 S0–S2 中Na +的特异性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号