Cell Reports ( IF 7.5 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.celrep.2020.107839 Goonho Park 1 , Hoang S Nhan 1 , Sheue-Houy Tyan 1 , Yusuke Kawakatsu 1 , Carolyn Zhang 1 , Mario Navarro 2 , Edward H Koo 3
|
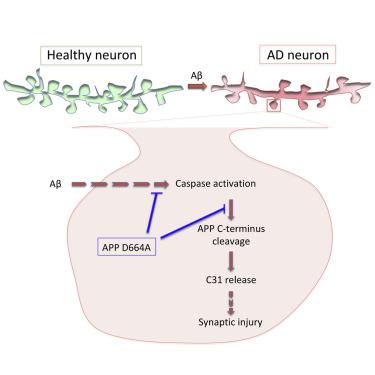
Amyloid β-protein (Aβ) toxicity is hypothesized to play a seminal role in Alzheimer’s disease (AD) pathogenesis. However, it remains unclear how Aβ causes synaptic dysfunction and synapse loss. We hypothesize that one mechanism of Aβ-induced synaptic injury is related to the cleavage of amyloid β precursor protein (APP) at position D664 by caspases that release the putatively cytotoxic C31 peptide. In organotypic slice cultures derived from mice with a knock-in mutation in the APP gene (APP D664A) to inhibit caspase cleavage, Aβ-induced synaptic injury is markedly reduced in two models of Aβ toxicity. Loss of dendritic spines is also attenuated in mice treated with caspase inhibitors. Importantly, the time-dependent dendritic spine loss is correlated with localized activation of caspase-3 but is absent in APP D664A cultures. We propose that the APP cytosolic domain plays an essential role in Aβ-induced synaptic damage in the injury pathway mediated by localized caspase activation.
中文翻译:

Caspase激活和Caspase介导的APP切割与淀粉样蛋白诱导的阿尔茨海默氏病引起的突触损失有关。
据推测淀粉样β蛋白(Aβ)毒性在阿尔茨海默氏病(AD)发病机理中起重要作用。但是,尚不清楚Aβ如何引起突触功能障碍和突触丧失。我们假设Aβ诱导的突触损伤的一种机制与释放假定的细胞毒性C31肽的胱天蛋白酶在D664位的淀粉样β前体蛋白(APP)的切割有关。在源自具有APP基因(APP D664A)的敲入突变以抑制caspase裂解的小鼠的器官型切片培养物中,在两种Aβ毒性模型中,Aβ诱导的突触损伤明显减少。在用半胱天冬酶抑制剂治疗的小鼠中,树突棘的损失也被减轻。重要的是,时间依赖性树突棘损失与caspase-3的局部活化有关,但在APP D664A培养物中则不存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号