Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-06-30 , DOI: 10.1016/j.apsb.2020.06.014 Yang Liu , Xiaojia Liu , Na Zhang , Mingxiao Yin , Jingwen Dong , Qingxuan Zeng , Genxiang Mao , Danqing Song , Lu Liu , Hongbin Deng
|
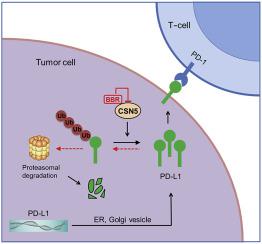
Programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) blocking therapy has become a major pillar of cancer immunotherapy. Compared with antibodies targeting, small-molecule checkpoint inhibitors which have favorable pharmacokinetics are urgently needed. Here we identified berberine (BBR), a proven anti-inflammation drug, as a negative regulator of PD-L1 from a set of traditional Chinese medicine (TCM) chemical monomers. BBR enhanced the sensitivity of tumour cells to co-cultured T-cells by decreasing the level of PD-L1 in cancer cells. In addition, BBR exerted its antitumor effect in Lewis tumor xenograft mice through enhancing tumor-infiltrating T-cell immunity and attenuating the activation of immunosuppressive myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs). BBR triggered PD-L1 degradation through ubiquitin (Ub)/proteasome-dependent pathway. Remarkably, BBR selectively bound to the glutamic acid 76 of constitutive photomorphogenic-9 signalosome 5 (CSN5) and inhibited PD-1/PD-L1 axis through its deubiquitination activity, resulting in ubiquitination and degradation of PD-L1. Our data reveals a previously unrecognized antitumor mechanism of BBR, suggesting BBR is small-molecule immune checkpoint inhibitor for cancer treatment.
中文翻译:

小碱通过抑制CSN5的去泛素化活性来减少癌细胞PD-L1的表达并促进抗肿瘤免疫
程序性细胞死亡1(PD-1)/程序性细胞死亡配体1(PD-L1)阻断疗法已成为癌症免疫疗法的主要支柱。与靶向抗体相比,迫切需要具有良好药代动力学的小分子检查点抑制剂。在这里,我们从一组中药(TCM)化学单体中鉴定出一种经过验证的抗发炎药小ber碱(BBR)作为PD-L1的负调节剂。BBR通过降低癌细胞中PD-L1的水平来增强肿瘤细胞对共培养T细胞的敏感性。此外,BBR通过增强肿瘤浸润性T细胞免疫力并减弱免疫抑制性髓样衍生抑制细胞(MDSCs)和调节性T细胞(Tregs)的激活,在Lewis肿瘤异种移植小鼠中发挥其抗肿瘤作用。BBR通过泛素(Ub)/蛋白酶体依赖性途径触发PD-L1降解。值得注意的是,BBR选择性结合组成型光形态9信号体5(CSN5)的谷氨酸76,并通过其去泛素化活性抑制PD-1 / PD-L1轴,导致泛素化和PD-L1降解。我们的数据揭示了先前无法识别的BBR抗肿瘤机制,表明BBR是用于癌症治疗的小分子免疫检查点抑制剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号