当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures and properties of side-chain liquid crystalline polynorbornenes containing an amide group: hydrogen bonding interactions and spacer length effects
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0py00586j Dong Shi 1, 2, 3, 4, 5 , Wen-Ying Chang 1, 2, 3, 4, 5 , Xiang-Kui Ren 6, 7, 8, 9 , Shuang Yang 1, 2, 3, 4, 5 , Er-Qiang Chen 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0py00586j Dong Shi 1, 2, 3, 4, 5 , Wen-Ying Chang 1, 2, 3, 4, 5 , Xiang-Kui Ren 6, 7, 8, 9 , Shuang Yang 1, 2, 3, 4, 5 , Er-Qiang Chen 1, 2, 3, 4, 5
Affiliation
|
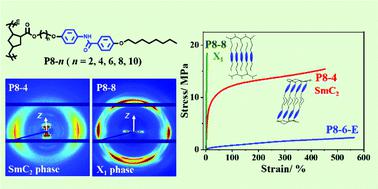
To investigate the structure–property relationship of side-chain liquid crystalline (LC) polymers with specific interactions, we synthesized a series of polynorbornene derivatives bearing benzanilide side-chains (denoted as P8-n, where n represents the number of methylene units in the spacer, and the number 8 indicates the side-chain tail of the octyl group). For comparison, a reference polynorbornene derivative (denoted as P8-6-E) was also synthesized by replacing the amide group in the rod-like mesogen with an ester group. It is found that with the polynorbornene main-chain the samples can exhibit rich LC behaviors, different from other benzanilide-containing polymers. P8-2 and P8-4 form a bilayer smectic C (SmC2) phase. On the other hand, with longer spacers, the molecules of P8-8 and P8-10 can pack into a highly ordered structure (denoted as X1). The X1 phase has an orthorhombic lattice with the side- and main-chains along the c- and b-axes, wherein the side-chains show interdigitated packing with some features of a crystal E (CrE) structure. For P8-6, some bilayer CrE domains may coexist with X1, resulting in a mixed phase of X1/E2. It is unveiled that the amide group at the center of benzanilide always tends to form hydrogen bonds, leading to the unique molecular packing of P8-ns which is dependent on the size matching between the spacer and tail. Moreover, weakening the hydrogen bonds in P8-ns (n ≥ 8) could induce a phase transition from X1 to X1/E2. Compared with the rather soft and ductile P8-6-E with a smectic B phase, P8-ns show much higher Young's moduli because of the presence of lateral hydrogen bonds. While those with the X1 phase are brittle, the P8-ns with a SmC2 structure exhibit better overall mechanical properties, rendering a breaking strain of ∼450%.
中文翻译:

含酰胺基的侧链液晶聚降冰片烯的结构和性质:氢键相互作用和间隔长度效应
为了研究具有特定相互作用的侧链液晶(LC)聚合物的结构与性质之间的关系,我们合成了一系列带有苯甲酰苯胺侧链的聚降冰片烯衍生物(表示为P8- n,其中n表示烯基中亚甲基单元的数目)间隔号,数字8表示辛基的侧链尾)。为了进行比较,还通过用酯基代替棒状液晶元中的酰胺基来合成参考聚降冰片烯衍生物(表示为P8-6-E)。发现与其他含苯甲酰苯胺的聚合物不同,使用聚降冰片烯主链可以显示出丰富的LC行为。P8-2和P8-4形成双层近晶C(SmC 2)相。另一方面,间隔物更长时,P8-8和P8-10分子可以堆积成高度有序的结构(表示为X 1)。X 1相具有正交的晶格,其侧链和主链沿c轴和b轴,其中,侧链显示出相互交叉的堆积,具有晶体E(CrE)结构的某些特征。对于P8-6,某些双层CrE域可能与X 1共存,导致X 1 / E 2混合相。揭示了苯甲酰苯胺中心的酰胺基总是倾向于形成氢键,导致P8- n s的独特分子堆积,这取决于间隔基和尾部之间的大小匹配。此外,削弱在氢键P8- Ñ S(Ñ ≥8)可诱导从X的相变1至X 1 / E 2。与相当柔软而有韧性相比P8-6-E具有近晶B相,P8- Ñ S显示由于横向氢键的存在的高得多的杨氏模量。X 1相的脆性,而P8- n具有SmC 2结构的S表现出更好的整体机械性能,断裂应变约为450%。
更新日期:2020-07-28
中文翻译:

含酰胺基的侧链液晶聚降冰片烯的结构和性质:氢键相互作用和间隔长度效应
为了研究具有特定相互作用的侧链液晶(LC)聚合物的结构与性质之间的关系,我们合成了一系列带有苯甲酰苯胺侧链的聚降冰片烯衍生物(表示为P8- n,其中n表示烯基中亚甲基单元的数目)间隔号,数字8表示辛基的侧链尾)。为了进行比较,还通过用酯基代替棒状液晶元中的酰胺基来合成参考聚降冰片烯衍生物(表示为P8-6-E)。发现与其他含苯甲酰苯胺的聚合物不同,使用聚降冰片烯主链可以显示出丰富的LC行为。P8-2和P8-4形成双层近晶C(SmC 2)相。另一方面,间隔物更长时,P8-8和P8-10分子可以堆积成高度有序的结构(表示为X 1)。X 1相具有正交的晶格,其侧链和主链沿c轴和b轴,其中,侧链显示出相互交叉的堆积,具有晶体E(CrE)结构的某些特征。对于P8-6,某些双层CrE域可能与X 1共存,导致X 1 / E 2混合相。揭示了苯甲酰苯胺中心的酰胺基总是倾向于形成氢键,导致P8- n s的独特分子堆积,这取决于间隔基和尾部之间的大小匹配。此外,削弱在氢键P8- Ñ S(Ñ ≥8)可诱导从X的相变1至X 1 / E 2。与相当柔软而有韧性相比P8-6-E具有近晶B相,P8- Ñ S显示由于横向氢键的存在的高得多的杨氏模量。X 1相的脆性,而P8- n具有SmC 2结构的S表现出更好的整体机械性能,断裂应变约为450%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号