当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alzheimer's disease-related amyloid β peptide causes structural disordering of lipids and changes the electric properties of a floating bilayer lipid membrane
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0na00292e Dusan Mrdenovic 1, 2 , Zhangfei Su 2 , Wlodzimierz Kutner 1, 3 , Jacek Lipkowski 2 , Piotr Pieta 1
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-06-29 , DOI: 10.1039/d0na00292e Dusan Mrdenovic 1, 2 , Zhangfei Su 2 , Wlodzimierz Kutner 1, 3 , Jacek Lipkowski 2 , Piotr Pieta 1
Affiliation
|
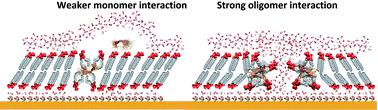
Neurodegeneration in Alzheimer's disease is associated with disruption of the neuronal cell membrane by the amyloid β (Aβ) peptide. However, the disruption mechanism and the resulting changes in membrane properties remain to be elucidated. To address this issue, herein the interaction of amyloid β monomers (AβMs) and amyloid β oligomers (AβOs) with a floating bilayer lipid membrane (fBLM) was studied using electrochemical and IR spectroscopy techniques. IR measurements showed that both Aβ forms interacted similarly with the hydrophobic membrane core (lipid acyl chains), causing conformational and orientational changes of the lipid acyl chains, thus decreasing acyl chain mobility and altering the lipid packing unit cell. In the presence of AβOs, these changes were more significant than those in the presence of AβMs. However, respective interactions of AβMs and AβOs with the membrane hydrophilic exterior (lipid heads) were quite different. AβMs dehydrated lipid heads without affecting their orientation while AβOs changed the orientation of lipid heads keeping their hydration level intact. Electrochemical measurements showed that only AβOs porated the fBLM, thus significantly changing the fBLM electrical properties. The present results provide new molecular-level insight into the mechanism of membrane destruction by AβOs and changes in the membrane properties.
中文翻译:

阿尔茨海默病相关的淀粉样蛋白 β 肽导致脂质结构紊乱并改变浮动双层脂质膜的电特性
阿尔茨海默病中的神经变性与淀粉样蛋白 β (Aβ) 肽对神经元细胞膜的破坏有关。然而,破坏机制和由此产生的膜特性变化仍有待阐明。为了解决这个问题,本文使用电化学和红外光谱技术研究了β淀粉样蛋白单体(AβMs)和β淀粉样蛋白寡聚体(AβOs)与浮动双层脂质膜(fBLM)的相互作用。IR 测量表明,两种 Aβ 形式与疏水膜核心(脂质酰基链)的相互作用相似,导致脂质酰基链的构象和取向变化,从而降低酰基链的流动性并改变脂质包装单元。在存在 AβOs 的情况下,这些变化比存在 AβMs 的情况更显着。然而,AβMs 和 AβOs 与膜亲水外部(脂质头)的各自相互作用是完全不同的。AβMs 脱水脂质头而不影响其方向,而 AβOs 改变脂质头的方向,保持其水合水平不变。电化学测量表明,只有 AβOs 使 fBLM 发生了变化,从而显着改变了 fBLM 的电性能。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。从而显着改变 fBLM 的电气特性。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。从而显着改变 fBLM 的电气特性。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。
更新日期:2020-08-11
中文翻译:

阿尔茨海默病相关的淀粉样蛋白 β 肽导致脂质结构紊乱并改变浮动双层脂质膜的电特性
阿尔茨海默病中的神经变性与淀粉样蛋白 β (Aβ) 肽对神经元细胞膜的破坏有关。然而,破坏机制和由此产生的膜特性变化仍有待阐明。为了解决这个问题,本文使用电化学和红外光谱技术研究了β淀粉样蛋白单体(AβMs)和β淀粉样蛋白寡聚体(AβOs)与浮动双层脂质膜(fBLM)的相互作用。IR 测量表明,两种 Aβ 形式与疏水膜核心(脂质酰基链)的相互作用相似,导致脂质酰基链的构象和取向变化,从而降低酰基链的流动性并改变脂质包装单元。在存在 AβOs 的情况下,这些变化比存在 AβMs 的情况更显着。然而,AβMs 和 AβOs 与膜亲水外部(脂质头)的各自相互作用是完全不同的。AβMs 脱水脂质头而不影响其方向,而 AβOs 改变脂质头的方向,保持其水合水平不变。电化学测量表明,只有 AβOs 使 fBLM 发生了变化,从而显着改变了 fBLM 的电性能。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。从而显着改变 fBLM 的电气特性。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。从而显着改变 fBLM 的电气特性。目前的结果为 AβO 破坏膜的机制和膜特性的变化提供了新的分子水平的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号