Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570180817666200117114716 Ameen Ali Abu-Hashem 1 , Khadiga Mohamed Abu-Zied 1 , Magdi Elsayed AbdelSalam Zaki 1 , Mohamed Fathy El-Shehry 2 , Hanem Mohamed Awad 3 , Mohammed Abdou Khedr 4
|
Background: Thienopyrimidine, triazole and thiazolidinone derivatives have recently gained attention due to their effective pharmacological activities. They show antioxidant, antitumor, antimicrobial, antiviral, anti-inflammatory and analgesic properties.
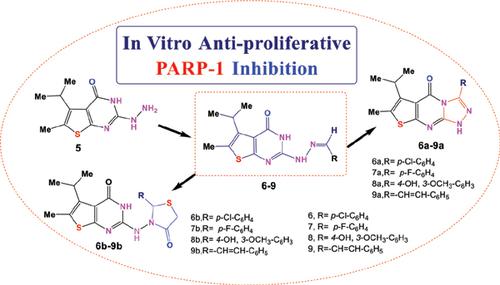
Objective: Synthesis of new ethyl 2-amino-4-isopropyl-5-methylthiophene-3-carboxylate (2) was used as a starting material to produce 2-mercapto-methylthienopyrimidinone (3), (4) and 2- hydrazinyl-methylthienopyrimidinone (5), through high yields and evaluating anticancer activities.
Methods: A series of novel Schiff's bases (6-9) were produced after treatment of (5) with aldehydes. Triazolopyrimidinones (6a, 7a, 8a, 9a) were produced from cyclization of benzylidene (6-9) using Br2 / AcOH or dry pyridine /Ac2O. Thiazolidinones (6b, 7b, 8b, 9b) were synthesized from benzylidene (6-9) with mercaptoacetic acid.
Results: All the compounds were synthesized in good yields (55-85%) in a regularly actual system under mild condition. The new compounds have been established by means of diverse spectroscopic ways as IR, NMR and MS. The newly synthesized compounds were evaluated for their antiproliferative activity against the breast MCF-7 carcinoma cell line. Compound (7) showed promising anticancer activity with IC50 of 6.9 μM, and 40.8% of antioxidant effect as DPPH inhibition. Molecular docking of (7) showed ΔG values of-20.54 kcal/ mol and -25.60 kcal/ mol. Molecular dynamics simulation of (7) in complex with PARP-1 revealed RMSD of 3.00 Ǻ.
Conclusion: The QSAR study confirmed the presence of a relationship between anticancer activity and subdivided surface area descriptors with coefficient r2 = 0.98 with high predictive power.
中文翻译:

新型三唑,噻唑烷酮,-Thieno [2,3-d]嘧啶酮的计算研究与酶(PARP-1)抑制剂的设计,合成和抗癌潜力
背景:噻吩并嘧啶,三唑和噻唑烷酮衍生物由于其有效的药理活性最近受到关注。它们具有抗氧化,抗肿瘤,抗微生物,抗病毒,抗炎和止痛的特性。
目的:合成新的2-氨基-4-异丙基-5-甲基噻吩-3-羧酸乙酯(2)作为原料制备2-巯基-甲基噻吩并嘧啶酮(3),(4)和2-肼基-甲基噻吩并嘧啶酮(5)通过高产并评估抗癌活性。
方法:用醛处理(5)后,制得一系列新颖的席夫碱(6-9)。使用Br2 / AcOH或干吡啶/ Ac2O通过亚苄基(6-9)的环化反应制得三唑并嘧啶酮(6a,7a,8a,9a)。由亚苄基(6-9)与巯基乙酸合成噻唑烷酮(6b,7b,8b,9b)。
结果:所有化合物均在温和条件下以规则的实际系统以高收率(55-85%)合成。通过多种光谱方法,如IR,NMR和MS建立了新化合物。评价新合成的化合物对乳腺癌MCF-7癌细胞系的抗增殖活性。化合物(7)具有令人满意的抗癌活性,IC50为6.9μM,抗DPPH抑制作用为40.8%。(7)的分子对接显示出ΔG值为-20.54kcal / mol和-25.60kcal / mol。(7)与PARP-1配合物的分子动力学模拟显示RMSD为3.00。
结论:QSAR研究证实了抗癌活性与细分的表面指标之间的关系,系数r2 = 0.98,具有较高的预测能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号