Nature Chemistry ( IF 19.2 ) Pub Date : 2020-06-29 , DOI: 10.1038/s41557-020-0489-1 Hang-Fei Tu 1 , Pusu Yang 1 , Zi-Hua Lin 1 , Chao Zheng 1 , Shu-Li You 1
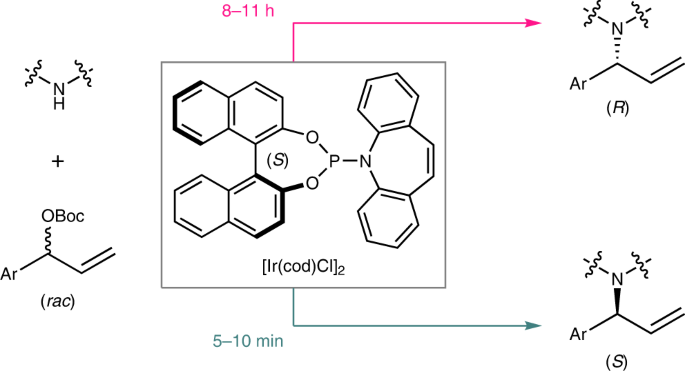
The preparation of both enantiomers of chiral molecules is among the most fundamental tasks in organic synthesis, medicinal chemistry and materials science. Achieving this goal typically requires reversing the absolute configuration of the chiral component employed in the reaction system that is being used. The task becomes challenging when the natural source of the chiral component is not available in both configurations. Herein, we report a time-dependent enantiodivergent synthesis, in which an Ir-catalysed allylic substitution reaction uses one catalyst sequentially to promote two kinetic resolution reactions, enabling the synthesis of both enantiomers of the product using the same enantiomer of a chiral catalyst. The appropriate permutation of individual reaction rates is essential for the isolation of the chiral products in opposite configurations with high enantiopurity when quenched at different reaction times. This work provides an alternative solution for the preparation of both enantiomers of chiral molecules.
中文翻译:

通过顺序动力学拆分随时间变化的对映体合成。
手性分子的两种对映体的制备是有机合成,药物化学和材料科学中最基本的任务之一。为了实现该目标,通常需要颠倒所使用的反应系统中所使用的手性组分的绝对构型。当两种配置中均没有手性组分的天然来源时,这项任务就变得具有挑战性。在本文中,我们报道了时间依赖性对映异构体合成,其中Ir催化的烯丙基取代反应顺序使用一种催化剂来促进两个动力学拆分反应,从而能够使用手性催化剂的相同对映体合成产物的两个对映体。当在不同的反应时间淬灭时,具有高对映体纯度的相反构型的手性产物的分离,各个反应速率的适当排列是必不可少的。这项工作为制备手性分子的两种对映异构体提供了另一种解决方案。












































 京公网安备 11010802027423号
京公网安备 11010802027423号