当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational changes of DNA repair glycosylase MutM triggered by DNA binding
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-23 , DOI: 10.1002/1873-3468.13876 Barbora Landová 1 , Jan Šilhán 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-07-23 , DOI: 10.1002/1873-3468.13876 Barbora Landová 1 , Jan Šilhán 1
Affiliation
|
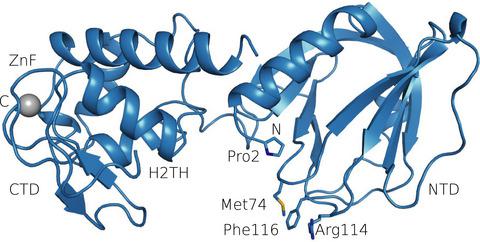
Bacterial MutM is a DNA repair glycosylase removing DNA damage generated from oxidative stress and, therefore, preventing mutations and genomic instability. MutM belongs to the Fpg/Nei family of prokaryotic enzymes sharing structural and functional similarities with their eukaryotic counterparts, for example, NEIL1–NEIL3. Here, we present two crystal structures of MutM from pathogenic Neisseria meningitidis: a MutM holoenzyme and MutM bound to DNA. The free enzyme exists in an open conformation, while upon binding to DNA, both the enzyme and DNA undergo substantial structural changes and domain rearrangement. Our data show that not only NEI glycosylases but also the MutMs undergo dramatic conformational changes. Moreover, crystallographic data support the previously published observations that MutM enzymes are rather flexible and dynamic molecules.
中文翻译:

DNA结合引发的DNA修复糖基化酶MutM的构象变化
Bacterial MutM 是一种 DNA 修复糖基化酶,可去除氧化应激产生的 DNA 损伤,从而防止突变和基因组不稳定。MutM 属于原核酶的 Fpg/Nei 家族,与它们的真核对应物(例如 NEIL1-NEIL3)共享结构和功能相似性。在这里,我们展示了来自致病性脑膜炎奈瑟菌的 MutM 的两种晶体结构:MutM 全酶和与 DNA 结合的 MutM。游离酶以开放构象存在,当与 DNA 结合时,酶和 DNA 都会发生实质性的结构变化和结构域重排。我们的数据表明,不仅 NEI 糖基化酶而且 MutM 都发生了显着的构象变化。此外,晶体学数据支持先前发表的观察结果,即 MutM 酶是相当灵活和动态的分子。
更新日期:2020-07-23
中文翻译:

DNA结合引发的DNA修复糖基化酶MutM的构象变化
Bacterial MutM 是一种 DNA 修复糖基化酶,可去除氧化应激产生的 DNA 损伤,从而防止突变和基因组不稳定。MutM 属于原核酶的 Fpg/Nei 家族,与它们的真核对应物(例如 NEIL1-NEIL3)共享结构和功能相似性。在这里,我们展示了来自致病性脑膜炎奈瑟菌的 MutM 的两种晶体结构:MutM 全酶和与 DNA 结合的 MutM。游离酶以开放构象存在,当与 DNA 结合时,酶和 DNA 都会发生实质性的结构变化和结构域重排。我们的数据表明,不仅 NEI 糖基化酶而且 MutM 都发生了显着的构象变化。此外,晶体学数据支持先前发表的观察结果,即 MutM 酶是相当灵活和动态的分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号