当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From very strong to inexistent Be-Be bonds in the interactions of Be2 with π-systems.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-06-29 , DOI: 10.1002/cphc.202000412 Eva Vos 1 , M Merced Montero-Campillo 1 , Inés Corral 1 , Manuel Yáñez 1 , Ibon Alkorta 2 , José Elguero 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-06-29 , DOI: 10.1002/cphc.202000412 Eva Vos 1 , M Merced Montero-Campillo 1 , Inés Corral 1 , Manuel Yáñez 1 , Ibon Alkorta 2 , José Elguero 2
Affiliation
|
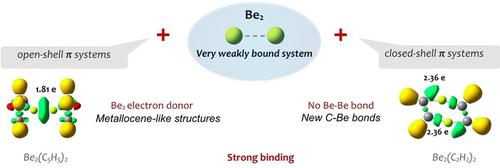
Isolated Be2 is a typical example of a weakly bound system, but interaction with other systems may give rise to surprising bonding features. The interactions between Be2 and a set of selected neutral CnHn (n=2–8) π‐systems have been analyzed through the use of G4 and G4MP2 ab initio methods, along with multireference CASPT2//CASPT2 calculations. Our results systematically show that the CnHn−Be2−CnHn clusters formed are always very stable. However, the nature of this interaction is completely different when the π‐system involved is a closed shell species (n=2, 4, 6, 8), or a radical (n=3, 5, 7). In the first case, the interaction does not occur with the π‐system as a whole, but with specific C centers yielding rather polar but strong C−Be bonds. Nonetheless, although the Be−Be distances in these complexes are similar to the ones in compounds with ultra‐strong Be−Be bonds, a close examination of their electron density distribution reveals that no Be−Be bonds exist. The situation is totally different when the interaction involves two π‐radicals, CnHn−Be2−CnHn (n=3, 5, 7). In these cases, a strong Be−Be bond is formed. Indeed, even though Be is electron deficient, the Be2 moiety behaves as an efficient electron donor towards the two π‐radicals, so that the different CnHn−Be2−CnHn (n=3, 5, 7) clusters are the result of the interaction between Be22+ and two L− anions. The characteristics of these two scenarios do not change when dealing with bicyclic π‐compounds, such as naphthalene and pentalene, because the interaction with the Be2 moiety is localized on one of the unsaturated cycles, the other being almost a spectator.
中文翻译:

在Be2与π系统的相互作用中,从非常强的Be-Be键不存在。
孤立的Be 2是弱结合系统的典型示例,但与其他系统的相互作用可能会产生令人惊讶的结合特征。通过使用G4和G4MP2从头算方法以及多参考CASPT2 // CASPT2计算,分析了Be 2与一组选定的中性C n H n(n = 2-8)π系统之间的相互作用。我们的结果系统地表明C n H n -Be 2 -C n H n形成的簇总是非常稳定的。但是,当涉及的π系统是封闭的壳物种(n = 2、4、6、8)或自由基(n = 3、5、7)时,这种相互作用的性质完全不同。在第一种情况下,与整个π系统不发生相互作用,而是与特定的C中心产生相当极性但牢固的C-Be键。尽管如此,尽管这些配合物中的Be-Be距离与具有超强Be-Be键的化合物中的Be-Be距离相似,但仔细检查它们的电子密度分布后发现,不存在Be-Be键。当相互作用涉及两个π自由基C n H n -Be 2 -C n H n时,情况完全不同。(n = 3、5、7)。在这些情况下,会形成牢固的Be-Be键。的确,即使Be缺乏电子,Be 2部分仍可作为朝向两个π自由基的有效电子供体,因此,不同的C n H n -Be 2 -C n H n(n = 3,5,7 )团簇是成为之间的相互作用的结果2个2+和两个L -阴离子。当处理双环π-化合物(例如萘和戊烯)时,这两种情况的特征不会改变,因为与Be 2部分的相互作用位于一个不饱和环上,另一个几乎是旁观者。
更新日期:2020-06-29
中文翻译:

在Be2与π系统的相互作用中,从非常强的Be-Be键不存在。
孤立的Be 2是弱结合系统的典型示例,但与其他系统的相互作用可能会产生令人惊讶的结合特征。通过使用G4和G4MP2从头算方法以及多参考CASPT2 // CASPT2计算,分析了Be 2与一组选定的中性C n H n(n = 2-8)π系统之间的相互作用。我们的结果系统地表明C n H n -Be 2 -C n H n形成的簇总是非常稳定的。但是,当涉及的π系统是封闭的壳物种(n = 2、4、6、8)或自由基(n = 3、5、7)时,这种相互作用的性质完全不同。在第一种情况下,与整个π系统不发生相互作用,而是与特定的C中心产生相当极性但牢固的C-Be键。尽管如此,尽管这些配合物中的Be-Be距离与具有超强Be-Be键的化合物中的Be-Be距离相似,但仔细检查它们的电子密度分布后发现,不存在Be-Be键。当相互作用涉及两个π自由基C n H n -Be 2 -C n H n时,情况完全不同。(n = 3、5、7)。在这些情况下,会形成牢固的Be-Be键。的确,即使Be缺乏电子,Be 2部分仍可作为朝向两个π自由基的有效电子供体,因此,不同的C n H n -Be 2 -C n H n(n = 3,5,7 )团簇是成为之间的相互作用的结果2个2+和两个L -阴离子。当处理双环π-化合物(例如萘和戊烯)时,这两种情况的特征不会改变,因为与Be 2部分的相互作用位于一个不饱和环上,另一个几乎是旁观者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号