当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Evaluation of Anticonvulsant Activity of Schiff Base of 1,4‐Benzodiazepine Amine
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-09-02 , DOI: 10.1002/cbdv.202000342 Pankaj R Nilkanth 1 , Sujit K Ghorai 2 , Arulmozhi Sathiyanarayanan 3 , Kiran Dhawale 3 , Tansir Ahamad 4 , Manoj B Gawande 5, 6 , Sharad N Shelke 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-09-02 , DOI: 10.1002/cbdv.202000342 Pankaj R Nilkanth 1 , Sujit K Ghorai 2 , Arulmozhi Sathiyanarayanan 3 , Kiran Dhawale 3 , Tansir Ahamad 4 , Manoj B Gawande 5, 6 , Sharad N Shelke 1
Affiliation
|
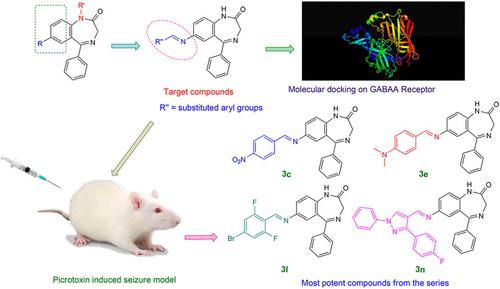
A variety of 1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one azomethines and 1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one benzamide were prepared, characterized and evaluated for the anticonvulsant activity in the rat using picrotoxin‐induced seizure model. The prepared 1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one azomethine derivatives emerged potentially anticonvulsant molecular scaffolds exemplified by compounds, 7‐{(E)‐[(4‐nitrophenyl)methylidene]amino}‐5‐phenyl‐1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one, 7‐[(E)‐{[4‐(dimethylamino)phenyl]methylidene}amino]‐5‐phenyl‐1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one, 7‐{(E)‐[(4‐bromo‐2,6‐difluorophenyl)methylidene]amino}‐5‐phenyl‐1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one and 7‐[(E)‐{[3‐(4‐fluorophenyl)‐1‐phenyl‐1H‐pyrazol‐4‐yl]methylidene}amino]‐5‐phenyl‐1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one. All these four compounds have shown substantial decrease in the wet dog shake numbers and grade of convulsions with respect to the standard drug diazepam. The most active compound, 7‐[(E)‐{[4‐(dimethylamino)phenyl]methylidene}amino]‐5‐phenyl‐1,3‐dihydro‐2H‐1,4‐benzodiazepin‐2‐one, exhibited 74 % protection against convulsion which was higher than the standard drug diazepam. Furthermore, to identify the binding mode of the interaction amongst the target analogs and binding site of the benzodiazepine receptor, molecular docking study and molecular dynamic simulation were carried out. Additionally, in silico pharmacokinetic and toxicity predictions of target compounds were carried out using AdmetSAR tool. Results of ADMET studies suggest that the pharmacokinetic parameters of all the target compounds were within the acceptable range to become a potential drug candidate as antiepileptic agents.
中文翻译:

1,4-苯二氮卓胺席夫碱抗惊厥活性的合成与评价
制备了多种 1,3-二氢-2H-1,4-苯二氮卓-2-酮偶氮甲碱和 1,3-二氢-2H-1,4-苯二氮卓-2-酮苯甲酰胺,表征并评估了抗惊厥活性在大鼠中使用 picrotoxin 诱导癫痫发作模型。制备的 1,3-dihydro-2H-1,4-benzodiazepin-2-one azomethine 衍生物出现了潜在的抗惊厥分子支架,例如化合物 7-{(E)-[(4-nitrophenyl)methylidene]amino}-5-苯基-1,3-二氢-2H-1,4-苯并二氮杂-2-one, 7-[(E)-{[4-(二甲基氨基)苯基]亚甲基}氨基]-5-苯基-1,3-二氢‐2H-1,4-benzodiazepin-2-one, 7-{(E)-[(4-bromo-2,6-difluorophenyl)methylidene]amino}-5-phenyl-1,3-dihydro-2H-1 ,4-苯二氮卓-2-one和7-[(E)-{[3-(4-氟苯基)-1-苯基-1H-吡唑-4-基]亚甲基}氨基]-5-苯基-1,3 -dihydro-2H-1,4-benzodiazepin-2-one。与标准药物地西泮相比,所有这四种化合物的湿狗摇晃次数和惊厥程度均显着降低。最活跃的化合物,7-[(E)-{[4-(二甲氨基)苯基]亚甲基}氨基]-5-苯基-1,3-二氢-2H-1,4-苯二氮卓-2-one,表现出74对惊厥的保护百分比高于标准药物地西泮。此外,为了确定目标类似物与苯二氮卓受体结合位点之间相互作用的结合模式,进行了分子对接研究和分子动力学模拟。此外,使用 AdmetSAR 工具对目标化合物进行了计算机模拟药代动力学和毒性预测。
更新日期:2020-09-02
中文翻译:

1,4-苯二氮卓胺席夫碱抗惊厥活性的合成与评价
制备了多种 1,3-二氢-2H-1,4-苯二氮卓-2-酮偶氮甲碱和 1,3-二氢-2H-1,4-苯二氮卓-2-酮苯甲酰胺,表征并评估了抗惊厥活性在大鼠中使用 picrotoxin 诱导癫痫发作模型。制备的 1,3-dihydro-2H-1,4-benzodiazepin-2-one azomethine 衍生物出现了潜在的抗惊厥分子支架,例如化合物 7-{(E)-[(4-nitrophenyl)methylidene]amino}-5-苯基-1,3-二氢-2H-1,4-苯并二氮杂-2-one, 7-[(E)-{[4-(二甲基氨基)苯基]亚甲基}氨基]-5-苯基-1,3-二氢‐2H-1,4-benzodiazepin-2-one, 7-{(E)-[(4-bromo-2,6-difluorophenyl)methylidene]amino}-5-phenyl-1,3-dihydro-2H-1 ,4-苯二氮卓-2-one和7-[(E)-{[3-(4-氟苯基)-1-苯基-1H-吡唑-4-基]亚甲基}氨基]-5-苯基-1,3 -dihydro-2H-1,4-benzodiazepin-2-one。与标准药物地西泮相比,所有这四种化合物的湿狗摇晃次数和惊厥程度均显着降低。最活跃的化合物,7-[(E)-{[4-(二甲氨基)苯基]亚甲基}氨基]-5-苯基-1,3-二氢-2H-1,4-苯二氮卓-2-one,表现出74对惊厥的保护百分比高于标准药物地西泮。此外,为了确定目标类似物与苯二氮卓受体结合位点之间相互作用的结合模式,进行了分子对接研究和分子动力学模拟。此外,使用 AdmetSAR 工具对目标化合物进行了计算机模拟药代动力学和毒性预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号