当前位置:
X-MOL 学术
›
Spectrochim. Acta B. At. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A comparison of different nanoscopic silver species with respect to their capacity to bind mercury from the gas-phase using total reflection X-ray fluorescence
Spectrochimica Acta Part B: Atomic Spectroscopy ( IF 3.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.sab.2020.105903 Sebastian Böttger , Joanna Kolny-Olesiak , Ursula E.A. Fittschen
Spectrochimica Acta Part B: Atomic Spectroscopy ( IF 3.2 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.sab.2020.105903 Sebastian Böttger , Joanna Kolny-Olesiak , Ursula E.A. Fittschen
|
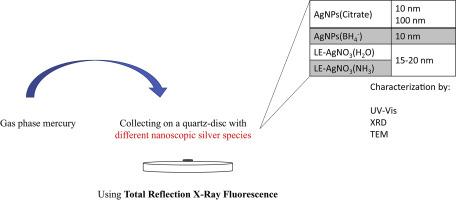
Abstract Mercury compounds like mercury chloride (HgCl2) were often used to protect cultural heritage specimens, e.g., herbaria and paintings from damage. Over time, Hg0 is formed by bacterial activity and released into the air. Here we present our study on the performance of different nanoscopic Ag species to collect airborne Hg, which is subsequently analyzed by total reflection X-ray fluorescence (TXRF). We studied silver nanoparticles (AgNPs) of different size, i.e., 10 nm and 100 nm AgNPs(Citrate) and particles with presumably weaker stabilizers, i.e., 10 nm AgNPs(borohydride(BH4−)) as well as species from light-exposed (LE) pure aqueous (LE-AgNO3(H2O)) and ammoniacal silver nitrate solutions (LE-AgNO3(NH3)). Hg capture was highest for the LE-AgNO3(NH3) species with a ratio of Hg/Ag of 0.78 in the amalgam. The performance to capture Hg decreased from LE-AgNO3(NH3) to AgNPs(Citrate) in the following order LE-AgNO3(NH3) > LE-AgNO3(H2O) > 10 nm AgNPs(BH4−) > 10 nm AgNPs(Citrate) > 100 nm AgNPs(Citrate), the latter collected airborne Hg with a ratio of Hg/Ag of 0.0006. The relative standard deviation of the TXRF measurements of Hg collected from a saturated atmosphere (n = 3 and n = 5) was lowest with 9% for LE-AgNO3(H2O) and highest for the AgNPs(Citrate) with 38%. The AgNPs(BH4−) and AgNPs(Citrate) species were also used to study Hg capture from the gas-phase in concentrations between 0.0012 mg/m3 and 0.024 mg/m3 (European Union regulated threshold: 0.02 mg/m3; saturated Hg atmosphere: 13.6 mg/m3 at 20 °C). Detection limits were found to be 0.018 μg/m3 for AgNPs(BH4−) and AgNPs(Citrate).
中文翻译:

使用全反射 X 射线荧光比较不同纳米级银物质结合气相汞的能力
摘要 汞化合物如氯化汞 (HgCl2) 常用于保护文化遗产标本,例如植物标本馆和绘画免受损坏。随着时间的推移,Hg0 由细菌活动形成并释放到空气中。在这里,我们展示了我们对不同纳米级 Ag 物种收集空气中汞的性能的研究,随后通过全反射 X 射线荧光 (TXRF) 对其进行分析。我们研究了不同尺寸的银纳米颗粒 (AgNPs),即 10 nm 和 100 nm AgNPs(柠檬酸盐)和可能具有较弱稳定剂的颗粒,即 10 nm AgNPs(硼氢化物(BH4-))以及来自曝光的物种( LE) 纯水溶液 (LE-AgNO3(H2O)) 和氨硝酸银溶液 (LE-AgNO3(NH3))。LE-AgNO3(NH3) 物质的汞捕获量最高,汞合金中的 Hg/Ag 比率为 0.78。捕获 Hg 的性能按以下顺序从 LE-AgNO3(NH3) 降低到 AgNPs(柠檬酸盐) LE-AgNO3(NH3) > LE-AgNO3(H2O) > 10 nm AgNPs(BH4−) > 10 nm AgNPs(柠檬酸盐) > 100 nm AgNPs(柠檬酸盐),后者收集空气中的 Hg,Hg/Ag 的比率为 0.0006。从饱和大气(n = 3 和 n = 5)收集的汞的 TXRF 测量值的相对标准偏差最低,LE-AgNO3(H2O) 为 9%,AgNPs(柠檬酸盐)最高,为 38%。AgNPs(BH4−) 和 AgNPs(Citrate) 物种也用于研究从气相中捕获汞,浓度在 0.0012 mg/m3 和 0.024 mg/m3 之间(欧盟规定的阈值:0.02 mg/m3;饱和汞大气: 13.6 mg/m3 在 20 °C)。发现 AgNPs(BH4-)和 AgNPs(柠檬酸盐)的检测限为 0.018 μg/m3。
更新日期:2020-08-01
中文翻译:

使用全反射 X 射线荧光比较不同纳米级银物质结合气相汞的能力
摘要 汞化合物如氯化汞 (HgCl2) 常用于保护文化遗产标本,例如植物标本馆和绘画免受损坏。随着时间的推移,Hg0 由细菌活动形成并释放到空气中。在这里,我们展示了我们对不同纳米级 Ag 物种收集空气中汞的性能的研究,随后通过全反射 X 射线荧光 (TXRF) 对其进行分析。我们研究了不同尺寸的银纳米颗粒 (AgNPs),即 10 nm 和 100 nm AgNPs(柠檬酸盐)和可能具有较弱稳定剂的颗粒,即 10 nm AgNPs(硼氢化物(BH4-))以及来自曝光的物种( LE) 纯水溶液 (LE-AgNO3(H2O)) 和氨硝酸银溶液 (LE-AgNO3(NH3))。LE-AgNO3(NH3) 物质的汞捕获量最高,汞合金中的 Hg/Ag 比率为 0.78。捕获 Hg 的性能按以下顺序从 LE-AgNO3(NH3) 降低到 AgNPs(柠檬酸盐) LE-AgNO3(NH3) > LE-AgNO3(H2O) > 10 nm AgNPs(BH4−) > 10 nm AgNPs(柠檬酸盐) > 100 nm AgNPs(柠檬酸盐),后者收集空气中的 Hg,Hg/Ag 的比率为 0.0006。从饱和大气(n = 3 和 n = 5)收集的汞的 TXRF 测量值的相对标准偏差最低,LE-AgNO3(H2O) 为 9%,AgNPs(柠檬酸盐)最高,为 38%。AgNPs(BH4−) 和 AgNPs(Citrate) 物种也用于研究从气相中捕获汞,浓度在 0.0012 mg/m3 和 0.024 mg/m3 之间(欧盟规定的阈值:0.02 mg/m3;饱和汞大气: 13.6 mg/m3 在 20 °C)。发现 AgNPs(BH4-)和 AgNPs(柠檬酸盐)的检测限为 0.018 μg/m3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号