Polymer Degradation and Stability ( IF 6.3 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.polymdegradstab.2020.109279 Peng Wang , Ming Liu , Qichao Ran
|
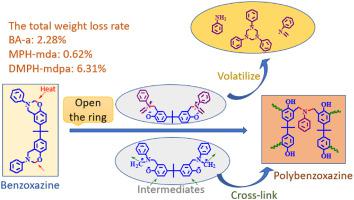
Based on the weight loss and small molecule volatiles released during the thermal curing process of three kinds of benzoxazines, bisphenol A/aniline type benzoxazine (BA-a), p-cresol/diamine type benzoxazine (MPH-mda) and 2,4-xylenol/4,4′-methylenebis-(2,6-diisopropyl) aniline type benzoxazine (DMPH-mdpa), their curing mechanisms and the weight-loss mechanisms were studied. The weight-loss ratios and structure changes of these benzoxazines during the curing process were studied by weighing method, Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). The volatiles released from these benzoxazines during different curing stages were collected by a self-made experimental device. The structures of these volatiles were characterized by FTIR and proton nuclear magnetic resonance spectroscopy (1H NMR). The results showed that the mixtures of imine, triazine, aniline and Schiff base were released from the cracking of the intermediate during the thermal curing of BA-a. Compared with BA-a, the weight loss of MPH-mda was smaller, and its volatile was a small amount of stable diamine type Schiff base. For DMPH-mdpa, because all active reaction points on benzene ring were occupied by alkyl groups, it cannot form effective polymer structures after ring-opening reactions, which led to that DMPH-mdpa had the most weight loss during the curing process, and mainly generated phenol type volatiles.
中文翻译:

苯并恶嗪热固化过程中的固化及减重机理研究
基于三种苯并恶嗪热固化过程中的重量损失和小分子挥发物,双酚A /苯胺型苯并恶嗪(BA-a),对甲酚/二胺型苯并恶嗪(MPH-mda)和2,4-研究了二甲苯酚/ 4,4'-亚甲基双-(2,6-二异丙基)苯胺型苯并恶嗪(DMPH-mdpa)的固化机理和减重机理。通过称量法,傅立叶变换红外光谱(FTIR)和差示扫描量热法(DSC)研究了这些苯并恶嗪在固化过程中的失重率和结构变化。通过自制的实验装置收集在不同固化阶段从这些苯并恶嗪释放的挥发物。这些挥发物的结构通过FTIR和质子核磁共振波谱(1 H NMR)。结果表明,在BA-a的热固化过程中,亚胺,三嗪,苯胺和席夫碱的混合物从中间体的裂化中释放出来。与BA-a相比,MPH-mda的重量损失较小,其挥发物为少量的稳定的二胺型席夫碱。对于DMPH-mdpa,由于苯环上的所有活性反应点都被烷基占据,因此开环反应后不能形成有效的聚合物结构,这导致DMPH-mdpa在固化过程中的失重最多,主要是产生酚类挥发物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号