Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.jhazmat.2020.123334 Ming-Xin Xu 1 , Han-Xiao Wang 1 , Hao-Dong Ouyang 1 , Li Zhao 1 , Qiang Lu 1
|
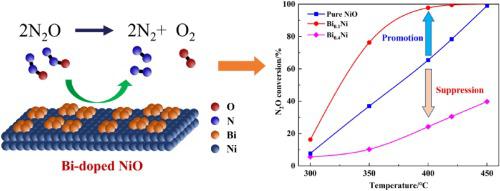
Direct catalytic decomposition is a promising technology to control the emission of nitrous oxide (N2O) during fossil fuel combustion and various chemical industries. In this study, a series of NiO catalysts modified with different metal oxides (MaNiOb) were prepared by the co-precipitation method and employed for the direct catalytic decomposition of N2O. Bismuth (Bi) species was confirmed to be the most optimal promoter and the Bi0.1NiO1.15 catalyst with a Bi/Ni molar ratio of 0.1 exhibited the best activity over the temperature range of 300−450 °C. The addition of Bi species also promoted the steam resistance capability of the NiO catalyst. Moreover, the physicochemical properties of pure and Bi-modified NiO catalysts were further determined by several characterization methods. The surface areas and capacity of oxygen adsorption/desorption over the catalyst were noticeably improved with the doping of Bi species. Besides, the presence of doped-Bi facilitated the creation of both Ni3+ and surface oxygen vacancies on NiO, which promoted the performance of N2O decomposition. Whereas, the excessive Bi species would accumulate to form large Bi2O3 grains, which diminished the surface areas and covered the active sites on the catalysts, leading to the rapid degradation of N2O catalytic decomposition.
中文翻译:

铋改性的NiO催化剂上N2O的直接催化分解。
直接催化分解是一种控制化石燃料燃烧和各种化学工业中一氧化二氮(N 2 O)排放的有前途的技术。在这项研究中,通过共沉淀法制备了一系列用不同金属氧化物改性的NiO催化剂(M a NiO b),并将其用于N 2 O的直接催化分解。最佳启动子和Bi 0.1 NiO 1.15Bi / Ni摩尔比为0.1的催化剂在300-450°C的温度范围内表现出最佳的活性。Bi物质的添加还提高了NiO催化剂的耐蒸汽性。此外,通过几种表征方法进一步确定了纯的和Bi改性的NiO催化剂的物理化学性质。掺入Bi物种显着提高了催化剂上的表面积和氧吸附/解吸的能力。此外,掺杂Bi的存在促进了NiO上Ni 3+和表面氧空位的产生,从而促进了N 2 O的分解性能。而过量的Bi会累积形成大的Bi 2 O 3。颗粒减少了表面积并覆盖了催化剂上的活性位,导致N 2 O催化分解迅速降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号