Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.jfluchem.2020.109596 Simonetta Orlandi , Marco Cavazzini , Silvia Capuani , Andrea Ciardello , Gianluca Pozzi
|
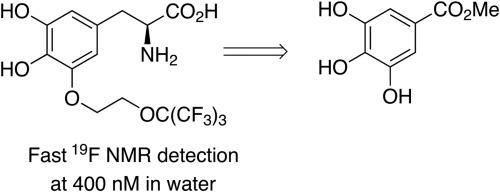
The robust multistep synthesis of a perfluoro-tert-butoxy (PFTB) tagged analogue of the non-proteinogenic amino acid 3,4-dihydroxy-L-phenylalanine (L-DOPA) via diastereoselective benzylation of Oppolzer’s sultam glycinate was developed. The new compound is characterized by a flexible alkoxy linker connecting PFTB to the biochemically and pharmacologically relevant L-DOPA scaffold, which facilitates the free motion of the fluorinated moiety. Therefore, the nine chemically equivalent fluorine atoms give rise to an intense and sharp 19F NMR singlet signal that is easily detected in an aqueous environment. In addition, the kinetics of 19F NMR relaxation processes in blood solution fit the requirements for the potential use of the new compound as a probe in 19F magnetic resonance imaging applications in translational clinical field.
中文翻译:

全氟叔丁氧基标记的L-DOPA类似物的合成和19 F NMR参数
开发了通过Oppolzer的苏丹草甘氨酸盐酸盐的非对映选择性苄基化方法,对非蛋白原氨基酸3,4-二羟基-L-苯丙氨酸(L-DOPA)进行全氟叔丁氧基(PFTB)标记的类似物的稳健多步合成。新化合物的特征是将PFTB连接到生物化学和药理学相关的L-DOPA支架的柔性烷氧基接头,该支架促进了氟化部分的自由运动。因此,这9个化学当量的氟原子产生了强烈而尖锐的19 F NMR单线态信号,该信号在水性环境中容易检测到。此外,血液溶液中19 F NMR弛豫过程的动力学符合将新化合物潜在用作探针的要求。19 F磁共振成像在翻译临床领域中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号