Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-06-29 , DOI: 10.1016/j.freeradbiomed.2020.06.015 Valesca Anschau 1 , Gerardo Ferrer-Sueta 2 , Rogerio Luis Aleixo-Silva 1 , Renata Bannitz Fernandes 1 , Carlos A Tairum 1 , Celisa Caldana Costa Tonoli 3 , Mario Tyago Murakami 3 , Marcos Antonio de Oliveira 4 , Luis Eduardo Soares Netto 1
|
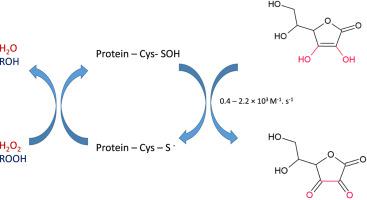
Sulfenic acids are the primary product of thiol oxidation by hydrogen peroxide and other oxidants. Several aspects of sulfenic acid formation through thiol oxidation were established recently. In contrast, the reduction of sulfenic acids is still scarcely investigated. Here, we characterized the kinetics of the reduction of sulfenic acids by ascorbate in several proteins. Initially, we described the crystal structure of our model protein (Tsa2-C170S). There are other Tsa2 structures in distinct redox states in public databases and all of them are decamers, with the peroxidatic cysteine very accessible to reductants, convenient features to investigate kinetics. We determined that the reaction between Tsa2-C170S-Cys-SOH and ascorbate proceeded with a rate constant of 1.40 ± 0.08 × 103 M-1 s-1 through a competition assay developed here, employing 2,6–dichlorophenol-indophenol (DCPIP). A series of peroxiredoxin enzymes (Prdx6 sub family) were also analyzed by this competition assay and we observed that the reduction of sulfenic acids by ascorbate was in the 0.4 – 2.2 × 103 M-1 s-1 range. We also evaluated the same reaction on glyceraldehyde 3-phosphate dehydrogenase and papain, as the reduction of their sulfenic acids by ascorbate were reported previously. Once again, the rate constants are in the 0.4 – 2.2 × 103 M-1 s-1 range. We also analyzed the reduction of Tsa2-C170S-SOH by ascorbate by a second, independent method, following hydrogen peroxide reduction through a specific electrode (ISO-HPO-2, World Precision Instruments) and employing a bi-substrate, steady state approach. The was 7.4 ± 0.07 × 103 M-1 s-1, which was in the same order of magnitude as the value obtained by the DCPIP competition assay. In conclusion, our data indicates that reduction of sulfenic acid in various proteins proceed at moderate rate and probably this reaction is more relevant in biological systems where ascorbate concentrations are high.
中文翻译:

通过蛋白质中的抗坏血酸还原亚磺酸,将硫醇依赖性连接到替代氧化还原途径。
亚磺酸是过氧化氢和其他氧化剂氧化硫醇的主要产物。最近建立了通过硫醇氧化形成亚磺酸的几个方面。相反,仍然很少研究亚磺酸的还原。在这里,我们表征了几种蛋白质中抗坏血酸还原亚磺酸的动力学。最初,我们描述了模型蛋白(Tsa2-C170S)的晶体结构。在公共数据库中,还存在其他处于不同氧化还原状态的Tsa2结构,并且它们都是十聚体,过氧化物半胱氨酸很容易被还原剂所利用,这是研究动力学的便利特征。我们确定Tsa2-C170S-Cys-SOH与抗坏血酸之间的反应以1.40±0.08×10 3 M -1的速率常数进行s -1通过此处开发的竞争测定法,采用2,6-二氯苯酚-吲哚酚(DCPIP)。通过该竞争分析法还分析了一系列过氧化物酶(Prdx6亚家族),我们观察到抗坏血酸对亚磺酸的还原度在0.4 – 2.2×10 3 M -1 s -1范围内。我们还评估了对甘油醛3-磷酸脱氢酶和木瓜蛋白酶的相同反应,因为先前已报道了抗坏血酸可将其亚磺酸还原。速率常数再次在0.4 – 2.2×10 3 M -1 s -1范围。我们还通过第二种独立方法分析了抗坏血酸对Tsa2-C170S-SOH的还原作用,该方法是通过特定电极(ISO-HPO-2,World Precision Instruments)将过氧化氢还原并采用双基板稳态方法。的为7.4±0.07×10 3 M -1 s -1,与通过DCPIP竞争分析获得的值处于同一数量级。总而言之,我们的数据表明,各种蛋白质中亚磺酸的还原反应均以中等速度进行,并且该反应可能在抗坏血酸浓度高的生物系统中更为相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号